For the following electrochemical cell Co(s)|Co2 (aq, 0.0155 M)||Ag (aq, 1.50 M)|Ag(s)
write the net cell equation. Phases are optional. Do not include the concentrations.
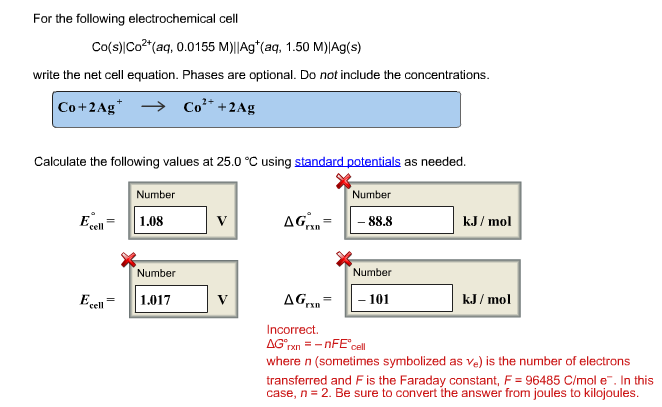
The Correct Answer and Explanation is :
The electrochemical cell you’ve provided is:
[ \text{Co(s)} | \text{Co}^{2+}(\text{aq}) || \text{Ag}(\text{aq}) | \text{Ag(s)} ]
The cell consists of two half-reactions: one at the cobalt electrode and one at the silver electrode. To write the net cell equation, we need to break the process down into the oxidation and reduction reactions occurring at each electrode.
Step 1: Identify the half-reactions.
- At the Co electrode (oxidation):
- Cobalt (Co) is oxidized to Co²⁺ (aq):
[
\text{Co(s)} \rightarrow \text{Co}^{2+}(\text{aq}) + 2e^-
]
This is the oxidation half-reaction, where cobalt loses two electrons.
- At the Ag electrode (reduction):
- Silver ions (Ag⁺) are reduced to silver metal (Ag):
[
\text{Ag}^+(\text{aq}) + e^- \rightarrow \text{Ag(s)}
]
This is the reduction half-reaction, where silver ions gain one electron.
Step 2: Balance the number of electrons in both half-reactions.
The oxidation half-reaction at the cobalt electrode involves 2 electrons, while the reduction half-reaction at the silver electrode involves only 1 electron. To balance the electrons, we need to multiply the silver half-reaction by 2:
[
2\left(\text{Ag}^+(\text{aq}) + e^- \rightarrow \text{Ag(s)}\right)
]
This gives the new half-reaction:
[
2\text{Ag}^+(\text{aq}) + 2e^- \rightarrow 2\text{Ag(s)}
]
Step 3: Combine the half-reactions to form the net equation.
Now, we combine the two half-reactions, ensuring the electrons cancel out:
[
\text{Co(s)} + 2\text{Ag}^+(\text{aq}) \rightarrow \text{Co}^{2+}(\text{aq}) + 2\text{Ag(s)}
]
Explanation:
This cell is a galvanic cell, where cobalt is being oxidized at the anode (the left side of the cell) and silver ions are being reduced at the cathode (the right side of the cell). In the overall reaction:
- Cobalt metal loses electrons and becomes Co²⁺ in solution, while silver ions gain electrons to form solid silver.
- The two half-reactions are connected by an external wire, which allows electrons to flow from the anode to the cathode, generating electrical energy.
- The flow of electrons is accompanied by the movement of ions in the solution, maintaining charge balance in the cell.
- The net cell reaction represents the complete transformation of cobalt and silver ions into their respective products, with the overall transfer of two electrons.