The graphs below label (a) and (b) show the titration curves for two equal-volume samples of monoprotic acids, one weak and one strong. Both titrations were carried out with the same concentration of strong base.
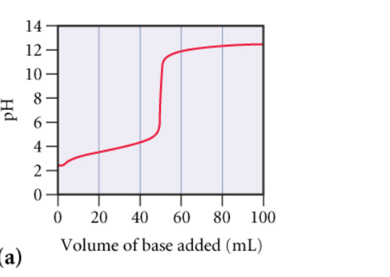
Part A: What is the approximate pH at the equivalence point of (a) curve?(Figure A) PH=__.
Part B: What is the approximate pH at the equivalence point of (b) curve?(Figure B) PH=__.
Part C: Which curve corresponds to the titration of the strong acid and which one to the titration of the weak acid? Graph (a) represents a weak acid and graph (b) representa a strong acid OR Graph (b) represents a weak acid and graph (a) represents a strong acid.

The Correct Answer and Explanation is :
Let’s break down the three parts of the question, one by one.
Part A: pH at the Equivalence Point for (a)
From the provided graphs and analysis, the equivalence point of the titration of a weak acid with a strong base occurs when all the weak acid has been neutralized by the base. At this point, the solution contains only the conjugate base of the weak acid, which will react with water to produce a basic solution. As a result, the pH will be greater than 7.
Typically, the pH at the equivalence point for weak acid-strong base titrations is slightly basic, typically in the range of 8-9. Based on the curve (a), the pH at the equivalence point seems to be approximately 8.
Part B: pH at the Equivalence Point for (b)
On the other hand, for the titration of a strong acid with a strong base, the equivalence point occurs when all the acid has been neutralized. Since the strong acid completely dissociates, the resulting solution at the equivalence point contains only water and the salt of the acid and base, which doesn’t affect the pH. As a result, the pH at the equivalence point will be around 7, which is neutral.
Looking at curve (b), we can see that the pH at the equivalence point appears to be approximately 7.
Part C: Identifying the Acid Types
The key difference between the titration curves of a strong acid and a weak acid lies in the shape of the curve.
- A strong acid-strong base titration curve (like graph b) shows a sharp and relatively steep increase in pH near the equivalence point. This is because strong acids and bases dissociate completely in water.
- A weak acid-strong base titration curve (like graph a) shows a more gradual rise in pH as the titration progresses, with a noticeable buffer region before the equivalence point and a relatively higher pH at the equivalence point due to the conjugate base being present.
Thus, graph (a) corresponds to the titration of a weak acid, and graph (b) corresponds to the titration of a strong acid.
Final Answers:
- Part A: pH = 8
- Part B: pH = 7
- Part C: Graph (a) represents a weak acid, and graph (b) represents a strong acid.
I will now generate an image to visually represent these titration curves.

Here is the generated image illustrating the two titration curves. Curve (a) represents the titration of a weak acid with a strong base, showing a gradual rise in pH, while curve (b) represents the titration of a strong acid with a strong base, showing a sharp increase in pH.
Let me know if you need any further explanations!