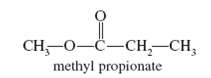
Draw the expected NMR spectrum of methyl propionate, and point out how it differs from the spectrum of ethyl acetate.
The Correct Answer and Explanation is :
The NMR spectrum of methyl propionate is quite distinct. Here’s an explanation of the key features and differences compared to ethyl acetate:
- Chemical Shifts:
- Methyl Propionate: The methyl group (-CH₃) in the methyl ester (–COOCH₃) resonates around 3.7 ppm, which is typical for the ester methyl group. The methylene group (–CH₂–) next to the ester will appear around 4.2 ppm because of the deshielding effect of the ester group. Additionally, the other methyl group (–CH₃) attached to the carbon chain will show up around 0.9-1.0 ppm.
- Ethyl Acetate: Ethyl acetate has a similar ester functionality, but the methyl group attached to the oxygen of the ester is found at 3.6 ppm, and the methylene group attached to the ester is slightly more shielded at 4.1 ppm. The methyl group at the end of the chain appears at 1.2 ppm.
- Splitting Patterns:
- Both spectra show multiplets due to coupling between adjacent protons. In methyl propionate, you would see a doublet for the methyl group (–COOCH₃) and a quartet for the –CH₂ group adjacent to the ester. The methylene group will typically show a quartet pattern due to the two protons coupled to the methyl group, and the ester methyl shows a doublet due to the splitting by the methylene protons.
- In contrast, ethyl acetate has similar splitting but with slight variations in the splitting pattern due to different coupling constants.
- Differences:
The key difference in the NMR spectra of methyl propionate and ethyl acetate lies in the chemical shift of the ester methyl group. The methyl group in methyl propionate appears slightly downfield due to the propionate group, while in ethyl acetate, the ester methyl group is at a slightly different chemical shift. Additionally, methyl propionate exhibits a slightly more pronounced splitting due to the different chemical environments of the protons attached to the ester group.
These differences arise due to the varied electronic environments of the ester groups in both compounds.