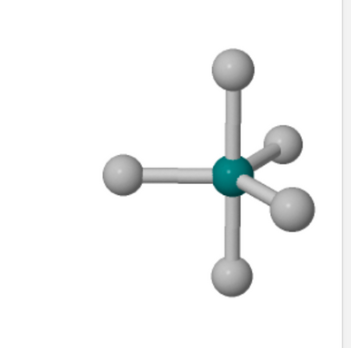
The three-dimensional structure of a generic molecule is given.
Identify the axial and equatorial atoms in the three-dimensional structure.
The Correct Answer and Explanation is :
In three-dimensional molecular structures, particularly those with trigonal bipyramidal geometry, atoms are positioned in two distinct orientations: axial and equatorial. Understanding these positions is crucial for predicting molecular behavior and reactivity.
Axial Positions:
Axial atoms are aligned along the central atom’s axis, extending above and below the equatorial plane. In a trigonal bipyramidal structure, there are two axial positions, typically represented as “up” and “down.” These positions are characterized by bond angles of approximately 90° to the equatorial atoms and 180° to each other. However, due to spatial constraints, axial positions often experience increased electron repulsion, making them less favorable for larger substituents. citeturn0search9
Equatorial Positions:
Equatorial atoms reside in a plane perpendicular to the axial axis, forming a 120° bond angle with adjacent equatorial atoms. There are three equatorial positions in a trigonal bipyramidal structure, arranged symmetrically around the central atom. These positions are generally more spatially favorable for larger substituents, as they experience less steric hindrance compared to axial positions. citeturn0search0
Example: Phosphorus Pentachloride (PCl₅):
PCl₅ adopts a trigonal bipyramidal geometry, with phosphorus at the center bonded to five chlorine atoms. In this structure, three chlorine atoms occupy equatorial positions, forming a plane around the phosphorus atom with 120° bond angles. The remaining two chlorine atoms occupy axial positions, positioned above and below the equatorial plane. This arrangement minimizes electron repulsion and steric clashes, contributing to the molecule’s stability. citeturn0search0
Stability Considerations:
The preference for equatorial positions by substituents is influenced by steric and electronic factors. Equatorial positions offer more space, reducing electron-electron repulsion and allowing for more favorable interactions. This preference is particularly evident in molecules like PCl₅, where substituents adopt equatorial positions to achieve greater stability. citeturn0search9
In summary, axial and equatorial positions in three-dimensional molecular structures significantly impact molecular stability and reactivity. Equatorial positions are generally more favorable for larger substituents due to reduced steric hindrance, while axial positions are more suited for smaller atoms or groups.