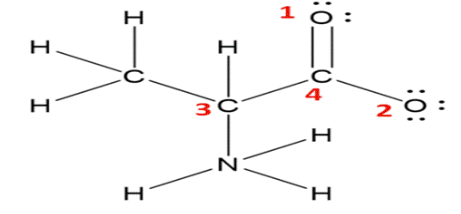
The Lewis structure for alanine is shown below. H 1 H H C 4 H 30 2 :0: ?. N H ?. a. What is the formal charge on the nitrogen atom? What are the formal charges on each of the oxygens, labeled 1 and 2 in red? b. What are the molecular geometries around the carbon atoms labeled 3 and 4?
The Correct Answer and Explanation is :
Alanine is an α-amino acid that serves as a building block for proteins. Its structure consists of a central carbon atom bonded to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a methyl group (-CH₃).
a. Formal Charges:
To determine the formal charges on the nitrogen and oxygen atoms, we apply the formal charge formula:
Formal Charge (FC) = Valence Electrons (VE) – Non-bonding Electrons (NBE) – (Bonding Electrons (BE) / 2)
- Nitrogen Atom:
- Valence Electrons (VE): Nitrogen is in Group 15 of the periodic table, so it has 5 valence electrons.
- Non-bonding Electrons (NBE): Nitrogen has one lone pair, contributing 2 non-bonding electrons.
- Bonding Electrons (BE): Nitrogen forms three single bonds (one with hydrogen and two with carbon), contributing 6 bonding electrons.
- Applying the formula: FC = 5 – 2 – (6 / 2) = 5 – 2 – 3 = 0 Therefore, the formal charge on the nitrogen atom is 0.
- Oxygen Atoms:
- Oxygen 1 (part of the carboxyl group):
- Valence Electrons (VE): Oxygen has 6 valence electrons.
- Non-bonding Electrons (NBE): Oxygen has two lone pairs, contributing 4 non-bonding electrons.
- Bonding Electrons (BE): Oxygen forms one double bond with carbon, contributing 4 bonding electrons.
- Applying the formula: FC = 6 – 4 – (4 / 2) = 6 – 4 – 2 = 0
- Oxygen 2 (part of the carboxyl group):
- Valence Electrons (VE): 6 valence electrons.
- Non-bonding Electrons (NBE): Three lone pairs, contributing 6 non-bonding electrons.
- Bonding Electrons (BE): Oxygen forms one single bond with carbon, contributing 2 bonding electrons.
- Applying the formula: FC = 6 – 6 – (2 / 2) = 6 – 6 – 1 = –1
b. Molecular Geometries:
- Carbon Atom 3 (α-carbon):
- This carbon is bonded to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom, and a methyl group (-CH₃).
- It has four substituents arranged tetrahedrally around it.
- Electron Geometry: Tetrahedral
- Molecular Geometry: Tetrahedral
- Carbon Atom 4 (carboxyl carbon):
- This carbon is double-bonded to oxygen 1 and single-bonded to oxygen 2.
- It has three substituents arranged trigonal planar around it.
- Electron Geometry: Trigonal Planar
- Molecular Geometry: Trigonal Planar
These geometries arise from the sp³ hybridization of carbon 3, leading to a tetrahedral arrangement, and the sp² hybridization of carbon 4, leading to a trigonal planar arrangement.