Draw the mirror image for each molecule:
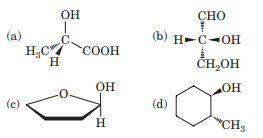
The Correct Answer and Explanation is :
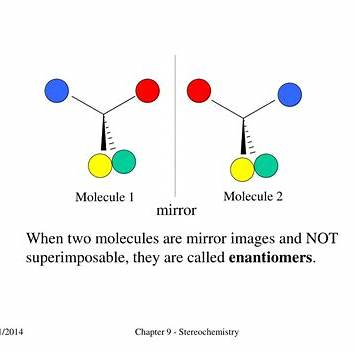
Chirality and Mirror Images:
Chirality refers to the geometric property wherein a molecule cannot be superimposed onto its mirror image, much like left and right hands. This non-superimposability arises when a central atom, typically carbon, is bonded to four distinct substituents, creating a chiral center. Such molecules exist as pairs of enantiomers, which are non-superimposable mirror images of each other.
Identifying Chiral Centers:
To determine if a molecule has a chiral center:
- Examine the Central Atom: Identify atoms bonded to four different groups.
- Assess Non-Superimposability: Determine if the mirror image of the molecule can be superimposed onto the original structure.
If both conditions are met, the molecule is chiral, and its mirror image is an enantiomer.
Example:
Consider 2-butanol:
CH3-CH(OH)-CH2-CH3The central carbon (C2) is attached to a hydroxyl group (OH), a methyl group (CH3), a hydrogen atom (H), and an ethyl group (CH2CH3). These are four distinct groups, making C2 a chiral center. Therefore, 2-butanol has a non-superimposable mirror image, classifying it as a chiral molecule with enantiomers.
Significance in Biology and Chemistry:
Chirality plays a crucial role in biological systems. Enzymes and receptors are often stereospecific, interacting only with specific enantiomers of molecules. This specificity underlies many biochemical processes and the effectiveness of pharmaceuticals, where one enantiomer may be therapeutic, and the other could be inactive or harmful.