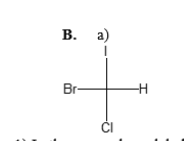
1) In the space above labeled b), draw the mirror image of molecule a.
2) Build molecule a and molecule b
3) Is molecule a superimposable on molecule b.
4) Are molecule a and molecule b the same molecule?
5) Name each molecule, a and b. What can you add to the names in order to distinguish molecule a from molecule b?
The Correct Answer and Explanation is :
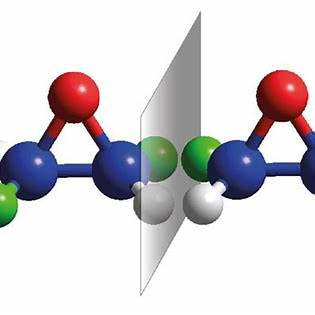
To address the questions regarding molecules ‘a’ and ‘b’:
1) Drawing the Mirror Image of Molecule ‘a’ in Space ‘b’:
Molecule ‘a’ is described as having a chiral center—a carbon atom bonded to four different groups. Its mirror image, molecule ‘b’, would have the same groups attached to the chiral center but arranged in a non-superimposable manner. This means that no matter how you rotate or translate molecule ‘b’, it cannot be made to overlap perfectly with molecule ‘a’.
2) Building Molecule ‘a’ and Molecule ‘b’:
Constructing both molecules involves arranging the four different substituents around a central carbon atom. For molecule ‘a’, the substituents are arranged in a specific spatial configuration. In molecule ‘b’, the mirror image configuration is adopted, resulting in a non-superimposable arrangement.
3) Is Molecule ‘a’ Superimposable on Molecule ‘b’?
No, molecule ‘a’ is not superimposable on molecule ‘b’. This non-superimposability indicates that the two molecules are distinct entities, known as enantiomers.
4) Are Molecule ‘a’ and Molecule ‘b’ the Same Molecule?
No, molecules ‘a’ and ‘b’ are not the same. They are enantiomers—mirror images of each other that cannot be superimposed. Enantiomers have identical physical and chemical properties in a symmetric environment but can exhibit different behaviors in chiral environments, such as interactions with other chiral molecules or polarized light.
5) Naming Each Molecule and Distinguishing Between Them:
Both molecules ‘a’ and ‘b’ would have the same molecular name based on their constituent atoms and bonds. To distinguish between them, the R/S system is used to denote their absolute configurations:
- Molecule ‘a’: Assigned the (R)-configuration.
- Molecule ‘b’: Assigned the (S)-configuration.
These designations are crucial in fields like pharmaceuticals, where the different enantiomers of a chiral drug can have vastly different effects in the body.
Illustrative Example:
Consider the molecule 2-bromobutane. It has a chiral center at the second carbon atom, with the following substituents:
- A bromine (Br) atom.
- A methyl (CH₃) group.
- An ethyl (C₂H₅) group.
- A hydrogen (H) atom.
The (R)-2-bromobutane and (S)-2-bromobutane are mirror images of each other and cannot be superimposed. Their different configurations can lead to different chemical behaviors, such as varying reactivity or interactions with biological systems.
Understanding the concepts of chirality, enantiomerism, and the R/S nomenclature is essential in stereochemistry, as these properties significantly influence the behavior and application of molecules in various chemical and biological contexts.