The barometric pressure in Nashville, Tennessee (elevation 597 ft or 182 m), averages about 29.3 inHg. Part A Convert this pressure to atm.
The correct answer and explanation is :
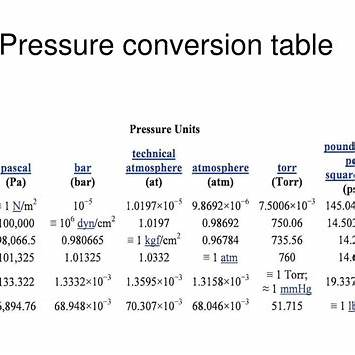
To convert a barometric pressure of 29.3 inches of mercury (inHg) to atmospheres (atm), we can use the conversion factor:
1 inHg = 0.033421 atm
Therefore, 29.3 inHg × 0.033421 atm/inHg = 0.9803 atm
Thus, the average barometric pressure in Nashville, Tennessee, is approximately 0.9803 atm.
Understanding the Conversion Process:
Barometric pressure, often measured in inches of mercury (inHg), indicates the atmospheric pressure at a specific location. An inch of mercury represents the pressure exerted by a 1-inch-high column of mercury at 0°C (32°F). This unit is commonly used in meteorology and aviation, particularly in the United States.
The standard atmosphere (atm) is another unit of pressure, defined as 101,325 Pascals (Pa). It approximates the average atmospheric pressure at sea level. Converting between inHg and atm requires understanding their relationship:
1 atm = 29.9213 inHg
This means that atmospheric pressure at sea level can support a mercury column approximately 29.9213 inches high. To convert inHg to atm, we use the reciprocal of this value:
1 inHg ≈ 1 / 29.9213 atm ≈ 0.033421 atm
Applying this conversion factor to the given pressure:
29.3 inHg × 0.033421 atm/inHg ≈ 0.9803 atm
Significance of Barometric Pressure:
Barometric pressure is crucial in weather forecasting and understanding atmospheric conditions. Higher pressures generally indicate fair weather, while falling pressures can signal approaching storms. Nashville’s average pressure of 29.3 inHg (0.9803 atm) is slightly below the standard sea-level pressure, which is reasonable given its elevation of 597 feet (182 meters). Elevation affects atmospheric pressure; higher altitudes have lower pressures due to the decreased weight of the air column above.
Practical Applications:
Understanding pressure conversions is vital in various fields:
- Meteorology: Accurate pressure readings help predict weather patterns.
- Aviation: Pilots rely on pressure measurements for altitude readings and flight safety.
- Medicine: Blood pressure measurements and respiratory therapies depend on precise pressure conversions.
In summary, converting barometric pressure from inHg to atm involves multiplying by the conversion factor 0.033421. This process allows for standardized pressure measurements across different scientific and practical applications.