Which of the statements below is the best hypothesis for the osmosis lab.
If a salt water solution is placed inside of the potato and plain tap water is placed outside of the potato then osmosis will occur and water molecules will move into the potato. And the level of water inside of the potato will rise.
If plain tap water is placed inside of the potato and a solution of salt water is placed outside of the potato then osmosis will occur and water molecules will move into the potato
If the the potato is soaked in salt water then it will gain weight by the water molecules moving into the potato cells. This is not osmosis
I think there should be no net movement of water molecules (osmosis) because the potato cells are already in an isotonic state.
The correct answer and explanation is :
The best hypothesis for the osmosis lab is:
“If plain tap water is placed inside of the potato and a solution of salt water is placed outside of the potato, then osmosis will occur and water molecules will move out of the potato.”
Explanation:
Osmosis is the movement of water molecules across a selectively permeable membrane from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration). In the case of a potato, its cells contain water and dissolved substances like sugars and salts.
If a potato is placed in saltwater, the solution outside the potato has a higher solute concentration than the water inside the potato cells. Because of this difference, water molecules will move out of the potato cells into the surrounding saltwater in an attempt to balance the concentrations. As a result, the potato will lose water, shrink, and become soft.
On the other hand, if the potato is placed in plain tap water, the water outside the potato has a higher water concentration (lower solute concentration) than the inside of the potato cells. In this case, water will move into the potato cells, causing them to swell and become firm.
The first hypothesis is incorrect because it suggests that water moves into the potato when the inside contains saltwater. However, the correct process is that water moves out of the potato when saltwater surrounds it.
The third hypothesis incorrectly states that soaking the potato in saltwater will cause it to gain weight, which contradicts osmosis. The fourth hypothesis assumes the potato is in an isotonic state, but this is usually not the case, as tap water and saltwater have different concentrations.
Osmosis in a Potato Experiment:
Here’s a visual representation of what happens in different solutions:
- In Saltwater: The potato loses water and shrinks.
- In Tap Water: The potato gains water and swells.
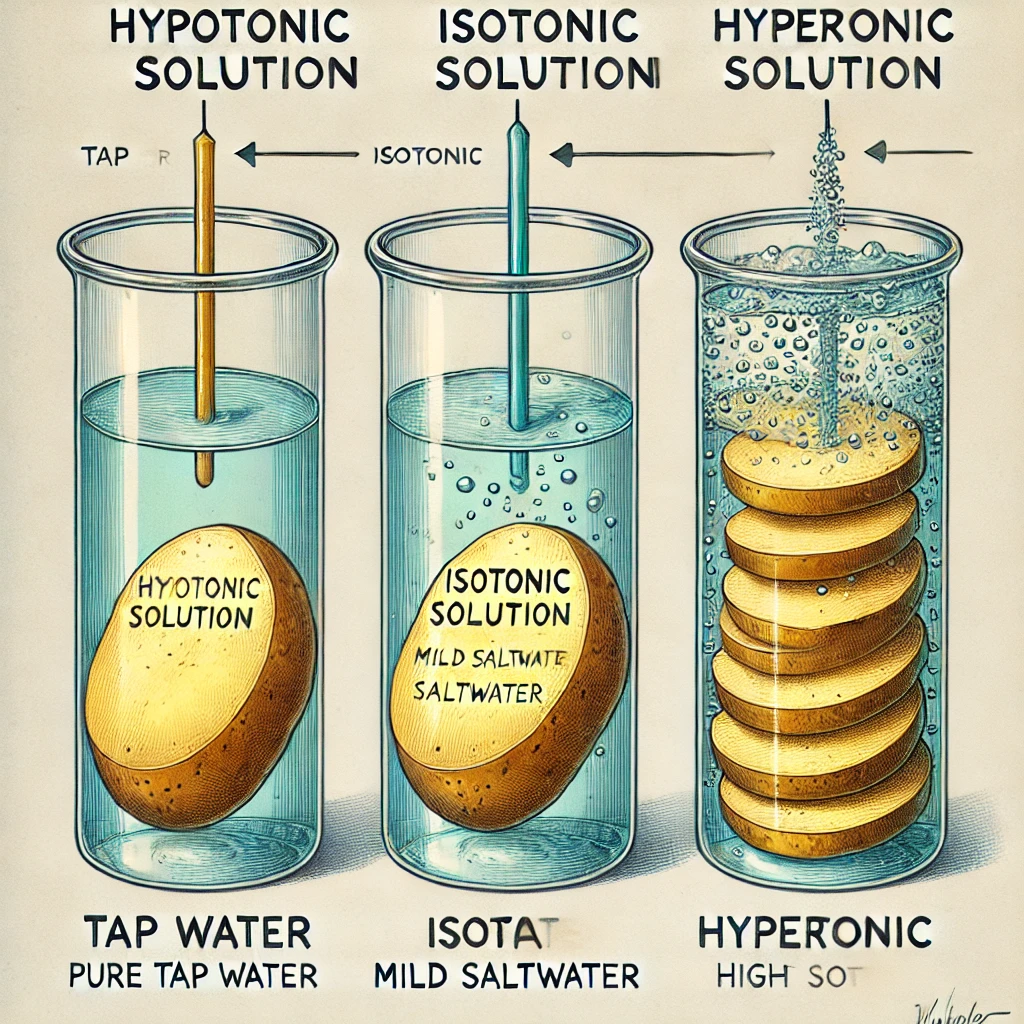
Here is a scientific illustration of osmosis in a potato experiment. The image visually represents the effects of different solutions (hypotonic, isotonic, and hypertonic) on potato slices, showing how water moves in or out of the cells.