Which of the following correctly states the impact of pressure on the behavior of real gases compared to ideal gases?
At low pressure, real gas particles do not exert any force on the container, so they exert less pressure than ideal gases.
At high pressure, real gas particles take up more of the available space, so their volume is greater than ideal gases.
At low pressure, real gas particles expand to fill the container so their volume cannot be measured compared to ideal gases.
At high pressure, real gas particles are squeezed together, so they take up less volume than ideal gases.
The correct answer and explanation is :
Correct Answer:
“At high pressure, real gas particles are squeezed together, so they take up less volume than ideal gases.”
Explanation:
The behavior of real gases deviates from ideal gas behavior, particularly under high pressure and low temperature conditions. The ideal gas law (PV = nRT) assumes that gas molecules have negligible volume and no intermolecular forces. However, real gases do not perfectly follow these assumptions.
1. High Pressure and Volume Deviation
At high pressures, gas molecules are forced closer together. This causes deviations from ideal gas behavior due to two key factors:
- Finite Volume of Gas Molecules: Unlike ideal gases (which assume particles have no volume), real gas molecules occupy space. As pressure increases, the free volume available for movement decreases, making the actual volume less than predicted by the ideal gas law.
- Intermolecular Attractions: At high pressures, gas molecules experience stronger attractive forces, which pull them closer together. This further reduces the space they occupy, causing the measured volume to be smaller than the ideal gas prediction.
2. Low Pressure and Real Gas Behavior
At low pressures, gas molecules are far apart, and the effect of intermolecular forces and molecular volume is negligible. Under these conditions, real gases behave more like ideal gases, following the ideal gas law closely.
Thus, the correct answer is that real gases take up less volume than ideal gases at high pressure because they are compressed due to intermolecular attractions and finite molecular size.
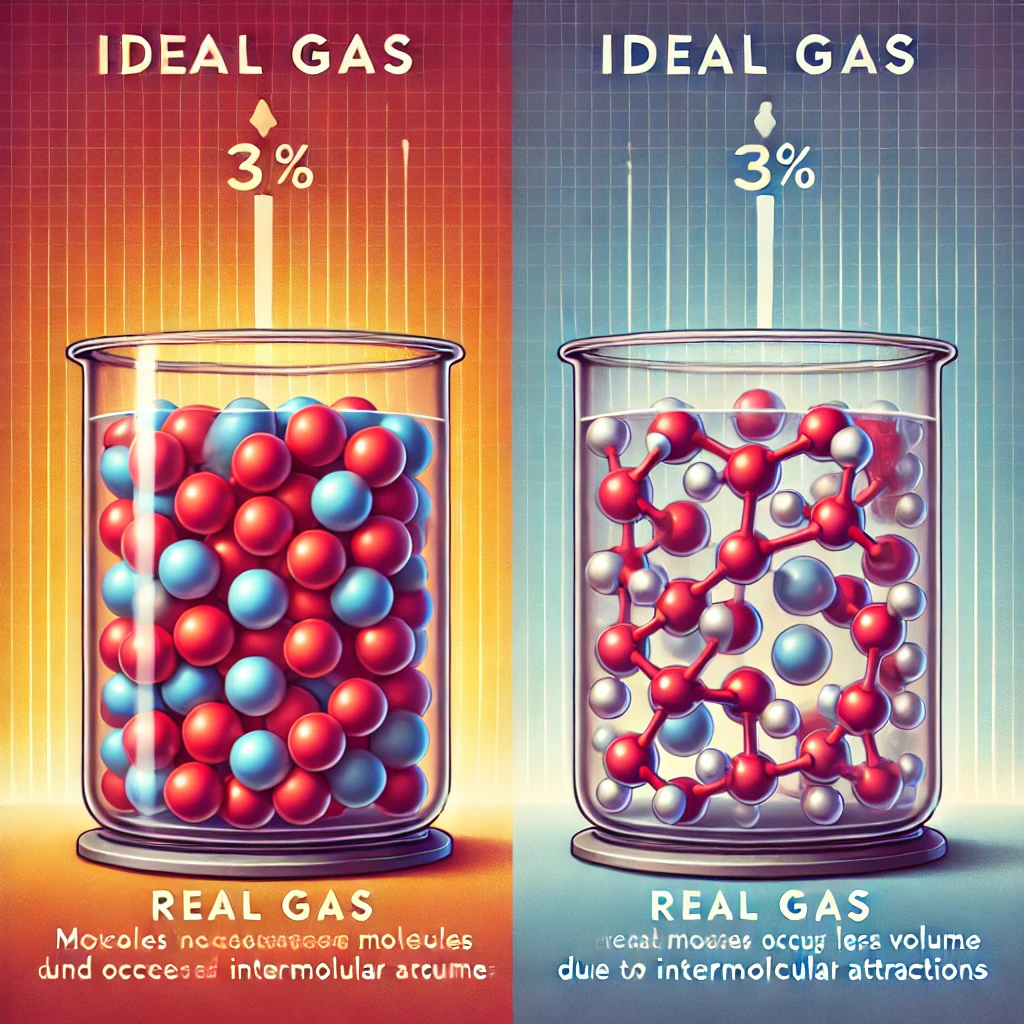
The image above illustrates the difference between real gas and ideal gas behavior under high pressure. It visually demonstrates how real gas molecules are compressed due to intermolecular forces, leading to a volume smaller than that predicted by the ideal gas law.