Draw the Lewis structure for chlorine tetrafluoride ion, indicate the formal charge and oxidation number of chlorine and predict ihe molecular shape of the ion using the VSEPR theory.
The correct answer and explanation is :
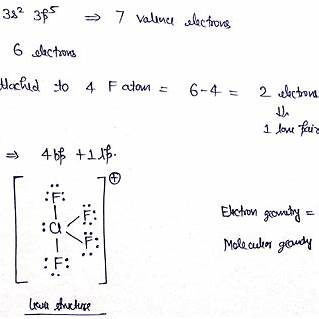
Chlorine tetrafluoride (ClF₄⁻) is a polyatomic ion consisting of a chlorine atom bonded to four fluorine atoms, carrying a negative charge.
Lewis Structure:
- Valence Electrons Calculation:
- Chlorine (Cl): 7 valence electrons
- Each Fluorine (F): 7 valence electrons × 4 = 28 valence electrons
- Total valence electrons: 7 (Cl) + 28 (F) + 1 (extra electron due to the negative charge) = 36 valence electrons
- Structure Formation:
- Place chlorine at the center, surrounded by four fluorine atoms.
- Connect each fluorine to chlorine with a single bond (2 electrons per bond).
- Distribute the remaining electrons to satisfy the octet rule for each atom.
Formal Charges:
- Chlorine:
- Valence electrons: 7
- Non-bonding electrons: 2 (one lone pair)
- Bonding electrons: 8 (4 bonds × 2 electrons)
- Formal charge: 7 – (2 + 8/2) = 0
- Fluorine:
- Valence electrons: 7
- Non-bonding electrons: 6
- Bonding electrons: 2
- Formal charge: 7 – (6 + 2/2) = 0
All atoms have a formal charge of zero, indicating a stable configuration.
Oxidation Number of Chlorine:
In ClF₄⁻, chlorine has an oxidation number of +5. This is determined by assigning -1 to each fluorine atom (since fluorine is more electronegative) and solving for chlorine’s oxidation state:
Oxidation number of Cl = +5
Molecular Shape Prediction (VSEPR Theory):
The central chlorine atom has four bonding pairs and one lone pair of electrons, totaling five regions of electron density. According to VSEPR theory, this arrangement leads to a trigonal bipyramidal electron geometry. However, the presence of the lone pair alters the molecular geometry to square pyramidal, with the lone pair occupying an equatorial position to minimize electron pair repulsion.
Summary:
- Lewis Structure: Chlorine at the center with single bonds to four fluorine atoms and one lone pair on chlorine.
- Formal Charges: All atoms have a formal charge of zero.
- Oxidation Number of Chlorine: +5.
- Molecular Geometry: Square pyramidal.