Molecules that have the same molecular formula, but different atomic connections are called constitutional isomers. Draw and name the five constitutional isomers of C6H14.
The correct answer and explanation is :
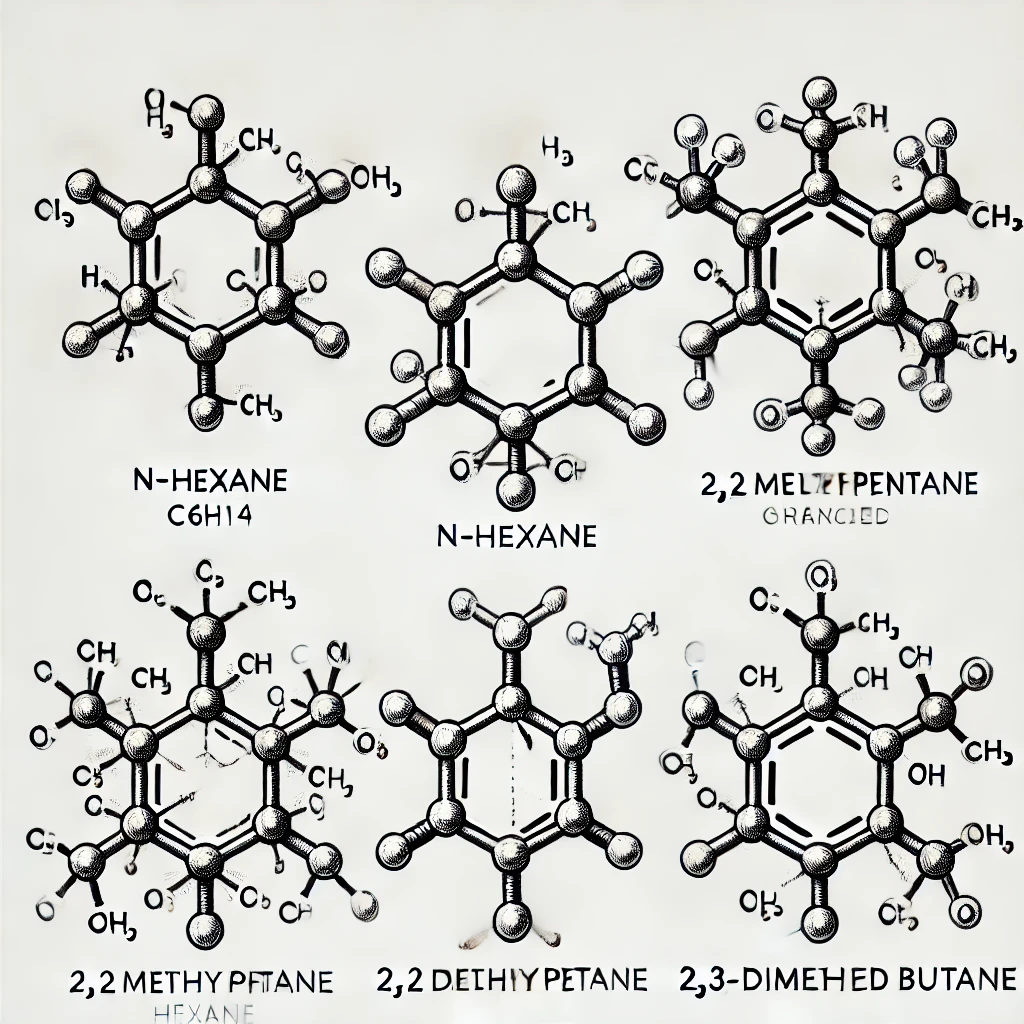
The image above shows the five constitutional isomers of hexane (C6H14), which are compounds with the same molecular formula but different atomic connections.
- n-Hexane: This is the straight-chain form of hexane. It has six carbon atoms connected in a linear arrangement, with each carbon bonded to enough hydrogen atoms to satisfy its valency of four.
- 2-Methylpentane: This isomer has a five-carbon chain, with a methyl group (-CH3) attached to the second carbon atom in the chain.
- 3-Methylpentane: Similar to 2-methylpentane, this isomer also has a five-carbon chain, but the methyl group is attached to the third carbon in the chain.
- 2,2-Dimethylbutane: This is a more highly branched isomer, with a four-carbon chain and two methyl groups attached to the second carbon in the chain.
- 2,3-Dimethylbutane: This isomer has a four-carbon chain as well, but the methyl groups are attached to the second and third carbons in the chain.
These are called constitutional isomers because, although they have the same molecular formula, their atoms are arranged differently, leading to distinct chemical structures and properties. The differences in the atomic connections result in variations in physical properties such as boiling points, densities, and solubility.