Which Of The Following Triatomic Molecules Will Have A Bond Angles Closest To 120°?
The correct answer and explanation is :
Here are some common triatomic molecules:
- CO₂ (Carbon Dioxide)
- SO₂ (Sulfur Dioxide)
- H₂O (Water)
- BF₃ (Boron Trifluoride, though it’s not a triatomic molecule, it’s planar and relevant for bond angle comparisons)
- O₃ (Ozone)
Correct Answer: SO₂ (Sulfur Dioxide)
Explanation:
The bond angle of a molecule depends on its molecular geometry, which is determined using VSEPR (Valence Shell Electron Pair Repulsion) Theory.
- CO₂ (Carbon Dioxide) – 180°
- Linear Geometry
- Carbon dioxide has no lone pairs on the central atom (C). The two oxygen atoms are arranged in a straight line, leading to a bond angle of 180°.
- H₂O (Water) – 104.5°
- Bent Geometry
- Oxygen in H₂O has two lone pairs, which strongly repel the hydrogen atoms, reducing the bond angle from the ideal 120° (trigonal planar) to about 104.5°.
- BF₃ (Boron Trifluoride) – 120° (Not a triatomic molecule)
- Trigonal Planar
- This molecule has three bonded atoms around the boron with no lone pairs, resulting in an exact 120° bond angle. However, this is not a triatomic molecule.
- O₃ (Ozone) – ~117°
- Bent Geometry
- Ozone has one lone pair on the central oxygen, leading to a slight deviation from 120°, making the bond angle about 117°.
- SO₂ (Sulfur Dioxide) – ~119° ✅
- Bent Geometry
- SO₂ has one lone pair on the central sulfur atom. Due to lone pair repulsion, the bond angle is slightly less than 120° but remains very close to it (~119°), making SO₂ the best answer.
Conclusion:
Among the given triatomic molecules, SO₂ has a bond angle closest to 120° due to its bent molecular shape with one lone pair on the central sulfur atom, following VSEPR theory.
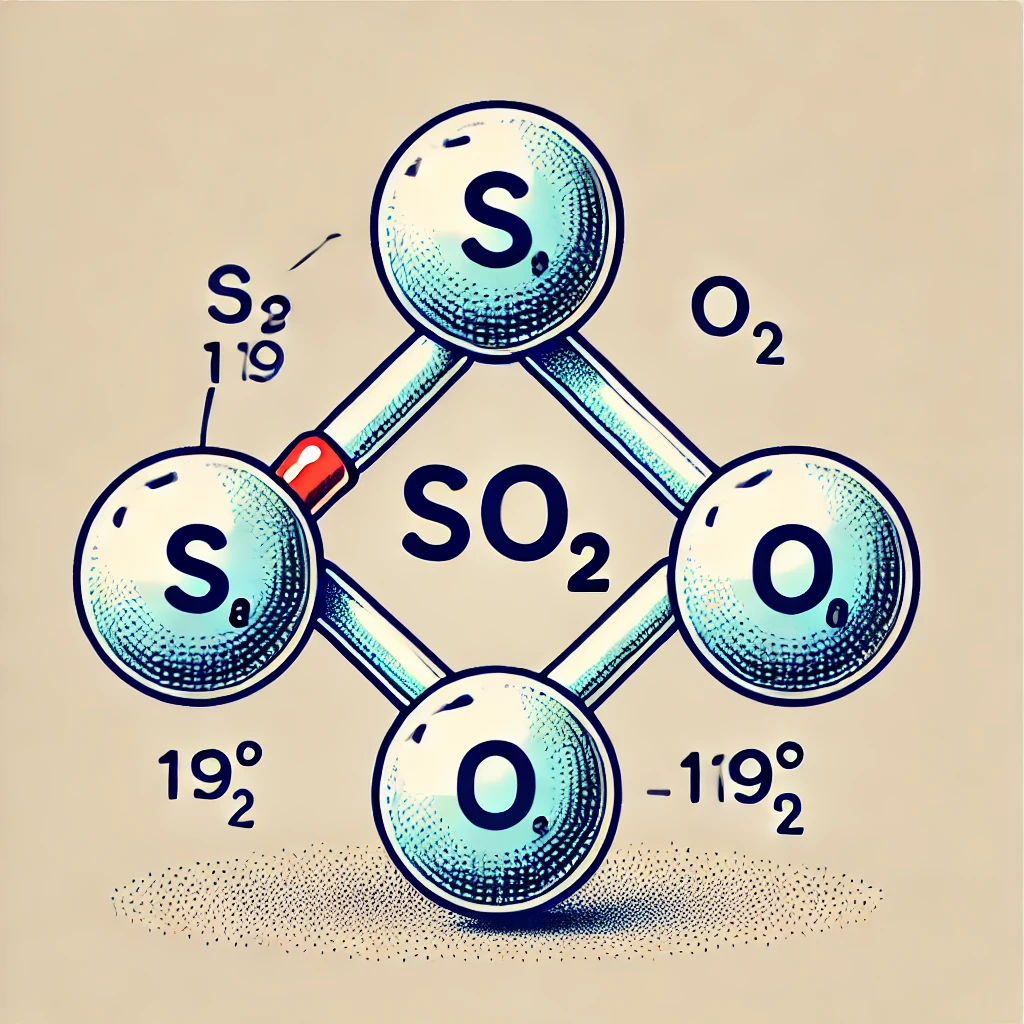
Here is the molecular structure of Sulfur Dioxide (SO₂), showing its bent shape and ~119° bond angle.