Draw the best Lewis dot structure for the anion IF4″ in the correct molecular geometry [Include formal charges, lone pair electrons and use dashed and solid wedge bonds if necessary in order to show correct geometry] 2. How many electron groups are present around the central atom and what is the electron group geometry? 3. What is the molecular geometry and ideal bond angles? 4. Is the molecule polar or nonpolar? If it is polar, draw a dipole moment arrow next to your structure to indicate the directionality of the dipole moment. None
The correct answer and explanation is:
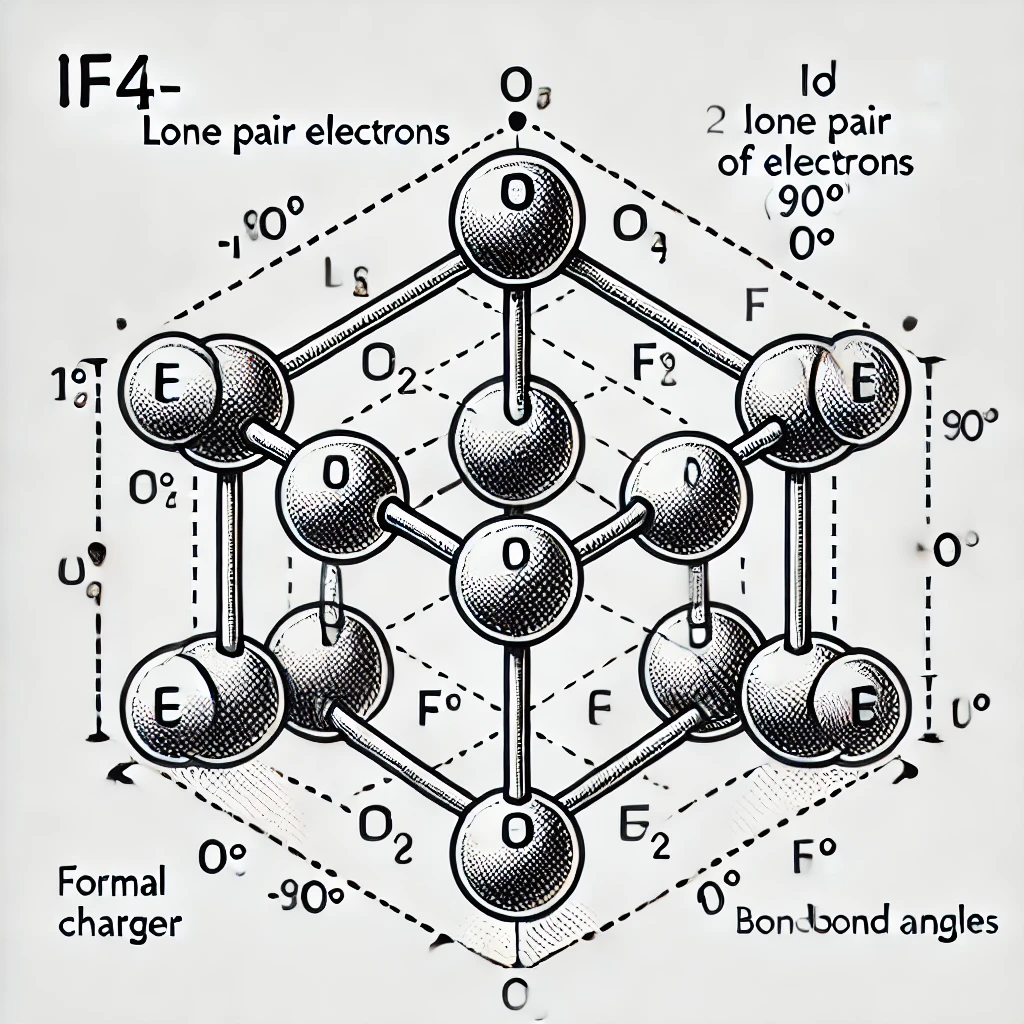
Lewis Structure of IF₄⁻ and Molecular Properties
1. Lewis Dot Structure
- The central atom is Iodine (I).
- Iodine has 7 valence electrons and gains one additional electron due to the negative charge, bringing the total to 8 valence electrons.
- Four Fluorine (F) atoms each contribute one electron, leading to a total of 36 valence electrons (8 from Iodine + 4×7 from Fluorine + 1 extra electron).
- Each Fluorine forms a single bond with Iodine, using 8 electrons (4 bonds × 2 electrons).
- The remaining 28 electrons are placed as lone pairs: 24 on the four Fluorine atoms and 4 (2 pairs) on Iodine.
- The formal charge of Iodine is -1, while all Fluorine atoms have a formal charge of 0.
2. Electron Groups and Electron Geometry
- There are 6 electron groups around Iodine (4 bonding pairs + 2 lone pairs).
- The electron group geometry is Octahedral.
3. Molecular Geometry and Bond Angles
- With two lone pairs, the molecular shape is Square Planar.
- The ideal bond angles are 90°.
4. Polarity and Dipole Moment
- Since all four fluorine atoms are symmetrically arranged in a square planar shape, the dipoles cancel out.
- The molecule is Nonpolar.
Now, I’ll generate the Lewis structure with correct geometry, formal charges, and lone pairs.
Here is the Lewis dot structure of the IF₄⁻ anion, showing the correct square planar geometry, lone pairs, formal charges, and bond angles.
Let me know if you need any modifications!