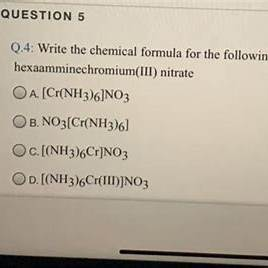
The correct formula of the compound whose name is hexaamminechromium(III) nitrate is
a. Cr(NO3)6
b. Cr(NH3)6
c. Cr(NO3)36
d. [Cr(NH3)6(NO3)3]
e. Cr(NH3)63
The correct formula for hexaamminechromium(III) nitrate is Cr(NH₃)₆₃, corresponding to option e.
Explanation:
In coordination chemistry, the nomenclature of complex compounds follows specific conventions:
- Naming the Ligands:
- Neutral Ligands: Neutral molecules acting as ligands are named as the molecule itself, sometimes with specific names (e.g., H₂O as ‘aqua’, NH₃ as ‘ammine’).
- Anionic Ligands: Anions are named by adding an ‘o’ suffix to their root name (e.g., Cl⁻ as ‘chloro’, NO₂⁻ as ‘nitro’).
- Naming the Central Metal Atom/Ion:
- The metal’s name is stated, followed by its oxidation state in Roman numerals within parentheses.
- Order in the Formula:
- The complex ion is enclosed in square brackets, with the metal symbol written first, followed by the ligands.
- Counterions (if any) are placed outside the brackets.
Applying these rules to hexaamminechromium(III) nitrate:
- Ligands: ‘Hexaammine’ indicates six ammonia (NH₃) molecules coordinated to the metal center.
- Central Metal: ‘Chromium(III)’ specifies a chromium ion with a +3 oxidation state.
- Counterion: ‘Nitrate’ denotes the presence of nitrate ions (NO₃⁻) as counterions.
The complex ion [Cr(NH₃)₆]³⁺ consists of a chromium ion surrounded by six ammonia ligands, resulting in a +3 charge. To balance this charge, three nitrate ions (NO₃⁻) are associated as counterions.
Thus, the chemical formula is Cr(NH₃)₆₃, aligning with option e.
This structure comprises the hexamminechromium(III) cation and three nitrate anions, ensuring electrical neutrality.
Understanding coordination compound nomenclature is crucial for interpreting and constructing chemical formulas accurately.