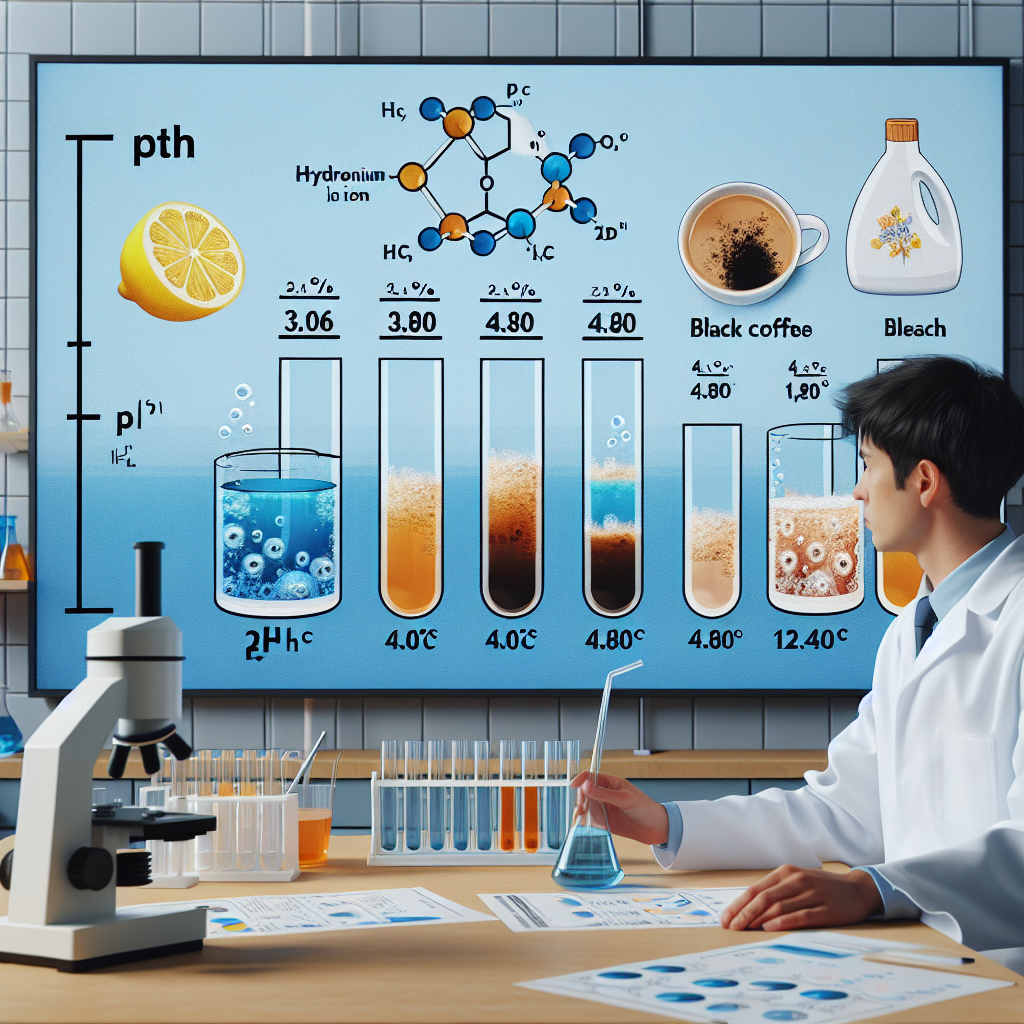
The pH of lemon juice was found to be approximately 3.06, while that of black coffee was approximately 4.80. The pH of bleach was measured to be approximately 12.40. All three solutions were at 25°C. The hydronium ion concentration of the most acidic solution is the following.
The correct answer and explanation is :
The hydronium ion concentration (([H_3O^+])) of a solution is related to its pH value by the following formula:
[
[H_3O^+] = 10^{-\text{pH}}
]
To find the hydronium ion concentration of the most acidic solution (which has the lowest pH value), we need to determine which solution has the lowest pH from the provided values.
pH Values:
- Lemon juice: pH = 3.06
- Black coffee: pH = 4.80
- Bleach: pH = 12.40
The most acidic solution is lemon juice, which has a pH of 3.06. To find the hydronium ion concentration, we apply the formula for ([H_3O^+]):
[
[H_3O^+] = 10^{-\text{pH}} = 10^{-3.06}
]
Now, let’s calculate this value:
[
[H_3O^+] = 10^{-3.06} \approx 8.51 \times 10^{-4} \, \text{M}
]
Explanation:
The pH scale is logarithmic, meaning that a decrease in pH by 1 unit corresponds to a tenfold increase in the concentration of hydronium ions. A solution with a lower pH has a higher concentration of hydronium ions, which makes it more acidic. Since lemon juice has the lowest pH (3.06) of the three solutions, it is the most acidic and has the highest concentration of hydronium ions.
In contrast, black coffee with a pH of 4.80 and bleach with a pH of 12.40 are less acidic, meaning their hydronium ion concentrations are lower. Specifically, black coffee has a hydronium ion concentration of ( 10^{-4.80} \approx 1.58 \times 10^{-5} \, \text{M} ), and bleach has a very low hydronium concentration of ( 10^{-12.40} \approx 4.00 \times 10^{-13} \, \text{M} ).
Thus, the hydronium ion concentration of the most acidic solution (lemon juice) is approximately ( 8.51 \times 10^{-4} \, \text{M} ).