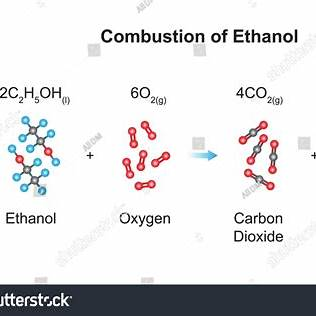
The combustion of ethanol, C2H5OH (molar mass = 46.1 g/mol), produces CO2 (molar mass = 44.0 g/mol) and H2O (molar mass = 18.0 g/mol). The density of ethanol is 0.789 g/mL, and the density of oxygen gas is 1.43 g/L. In one experiment, 17.6 mL of ethanol were combined with 10.5 L O2 and allowed to react.
a. Give the balanced chemical equation for the combustion of ethanol.
b. Determine the theoretical yield of CO2 in grams. The density of CO2 is 1.98 g/L.
c. How many grams of O2 will remain when the reaction is complete?
d. How many milliliters of C2H5OH will remain when the reaction is complete?
The correct answer and explanation is :
a. Balanced Chemical Equation for the Combustion of Ethanol
The combustion of ethanol, C₂H₅OH, occurs when ethanol reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). The balanced chemical equation is:
[
C_2H_5OH (l) + 3O_2 (g) \rightarrow 2CO_2 (g) + 3H_2O (l)
]
b. Theoretical Yield of CO₂ in Grams
Step 1: Convert the given amount of ethanol (C₂H₅OH) to moles.
We are given that 17.6 mL of ethanol were used, and the density of ethanol is 0.789 g/mL. First, calculate the mass of ethanol:
[
\text{Mass of ethanol} = 17.6 \, \text{mL} \times 0.789 \, \text{g/mL} = 13.9 \, \text{g}
]
Next, convert the mass of ethanol to moles using the molar mass of ethanol (46.1 g/mol):
[
\text{Moles of ethanol} = \frac{13.9 \, \text{g}}{46.1 \, \text{g/mol}} = 0.301 \, \text{mol}
]
Step 2: Determine the moles of CO₂ produced.
From the balanced chemical equation, we see that 1 mole of ethanol produces 2 moles of CO₂. Therefore, the moles of CO₂ produced are:
[
\text{Moles of CO₂} = 2 \times 0.301 \, \text{mol} = 0.602 \, \text{mol}
]
Step 3: Convert moles of CO₂ to grams.
To find the mass of CO₂, use the molar mass of CO₂ (44.0 g/mol):
[
\text{Mass of CO₂} = 0.602 \, \text{mol} \times 44.0 \, \text{g/mol} = 26.5 \, \text{g}
]
Thus, the theoretical yield of CO₂ is 26.5 grams.
c. Grams of O₂ that Will Remain
Step 1: Determine the moles of O₂ that reacted.
From the balanced equation, 1 mole of ethanol reacts with 3 moles of O₂. Therefore, the moles of O₂ required for the reaction are:
[
\text{Moles of O₂ required} = 3 \times 0.301 \, \text{mol} = 0.903 \, \text{mol}
]
Step 2: Convert the moles of O₂ to grams.
The molar mass of O₂ is 32.0 g/mol, so:
[
\text{Mass of O₂ required} = 0.903 \, \text{mol} \times 32.0 \, \text{g/mol} = 28.9 \, \text{g}
]
Step 3: Convert the volume of O₂ used to grams.
We are given that 10.5 L of O₂ were used. The density of O₂ is 1.43 g/L, so the mass of O₂ available is:
[
\text{Mass of O₂ available} = 10.5 \, \text{L} \times 1.43 \, \text{g/L} = 15.0 \, \text{g}
]
Step 4: Determine the remaining O₂.
Since only 15.0 g of O₂ were available, and 28.9 g were required, there will be no O₂ remaining. Instead, O₂ is the limiting reagent in this reaction.
d. Milliliters of Ethanol that Will Remain
Since the O₂ is the limiting reagent, all of the available O₂ will be consumed. The reaction requires 0.903 moles of O₂, which will react with 0.301 moles of ethanol. We already calculated that the moles of ethanol required are 0.301 mol, which is exactly the amount of ethanol available. Thus, all of the ethanol will be consumed, and there will be no ethanol remaining.
Image Representation of the Chemical Reaction Process