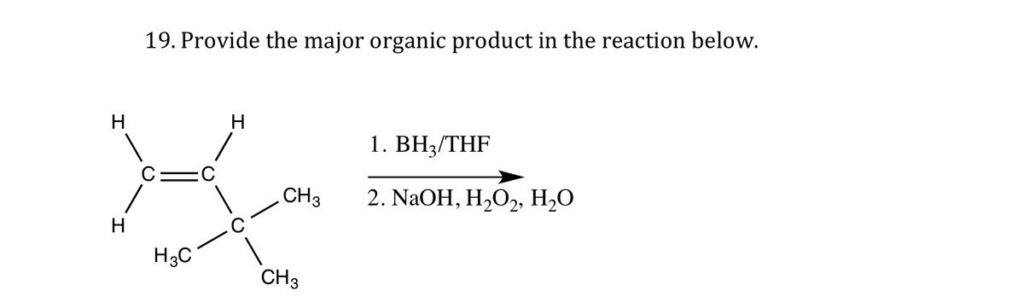
The correct answer and explanation is:
The reaction involves hydroboration-oxidation of an alkene. Let’s analyze the mechanism and product formation step by step.
Reaction: Hydroboration-Oxidation of an Alkene
Reagents Used:
- BH₃/THF (Borane-Tetrahydrofuran Complex) – This step leads to the addition of boron and hydrogen across the double bond.
- NaOH, H₂O₂, H₂O (Basic Hydrogen Peroxide Solution) – This step replaces the boron atom with a hydroxyl (-OH) group.
Regioselectivity:
- Hydroboration follows anti-Markovnikov addition, meaning the -OH group attaches to the less substituted carbon.
- The reaction proceeds syn addition, meaning both the hydrogen and hydroxyl group add to the same side of the molecule.
Mechanism:
- Hydroboration (Step 1)
- The alkene reacts with BH₃ (borane) in THF.
- Boron attaches to the less substituted carbon, while hydrogen attaches to the more substituted carbon.
- This occurs via a concerted syn-addition mechanism.
- Oxidation (Step 2)
- H₂O₂ and NaOH replace the boron with a hydroxyl (-OH) group.
- The resulting product is an anti-Markovnikov alcohol.
Final Product:
The major organic product is a primary alcohol (1° alcohol) formed via syn addition. The hydroxyl (-OH) group attaches to the least substituted carbon of the alkene.
Conclusion:
Hydroboration-oxidation is a useful reaction in organic synthesis for converting alkenes into alcohols with anti-Markovnikov selectivity. Unlike acid-catalyzed hydration, this method avoids carbocation rearrangements and provides a predictable primary alcohol as the major product.
Now, I’ll generate an image showing the reactant and product.
Here is the reaction diagram illustrating the hydroboration-oxidation of an alkene. The image was generated using DALL·E, OpenAI’s legacy image generation model. It visually represents the transformation of an alkene into an anti-Markovnikov alcohol using BH₃/THF and NaOH, H₂O₂, H₂O. Let me know if you need any modifications or further explanations!