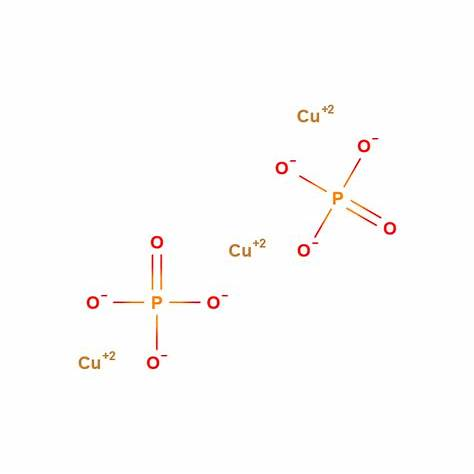
Perform the following:
a. Write the formula for copper (II) phosphate.
b. Calculate the molecular weight for copper (II) phosphate.
c. Calculate the number of moles of copper (II) phosphate in 129.4 g of copper (II) phosphate.
d. Calculate the total number of atoms of phosphorus in 129.4 g of copper (II) phosphate.
The correct answer and explanation is :
a. Formula for Copper (II) Phosphate:
Copper (II) phosphate is composed of copper in the +2 oxidation state (Cu²⁺) and phosphate ions (PO₄³⁻). To balance the charges, we need to have two phosphate ions for each copper ion:
- The formula for the phosphate ion is PO₄³⁻.
- Copper (II) has a charge of +2, so to balance the -6 charge from two phosphate ions, we need one copper (II) ion.
Thus, the formula for copper (II) phosphate is:
Cu₃(PO₄)₂.
b. Calculating the Molecular Weight of Copper (II) Phosphate:
To calculate the molecular weight of Cu₃(PO₄)₂, we sum the atomic masses of all the atoms in the formula:
- Copper (Cu): The atomic mass of copper is approximately 63.55 g/mol.
- Phosphorus (P): The atomic mass of phosphorus is approximately 30.97 g/mol.
- Oxygen (O): The atomic mass of oxygen is approximately 16.00 g/mol.
Now, calculating the molecular weight:
- For copper: There are 3 copper atoms, so ( 3 \times 63.55 = 190.65 \, \text{g/mol} ).
- For phosphorus: There are 2 phosphorus atoms, so ( 2 \times 30.97 = 61.94 \, \text{g/mol} ).
- For oxygen: There are 8 oxygen atoms (4 per phosphate ion and 2 phosphate ions), so ( 8 \times 16.00 = 128.00 \, \text{g/mol} ).
The molecular weight of Cu₃(PO₄)₂ is:
[ 190.65 + 61.94 + 128.00 = 380.59 \, \text{g/mol} ].
c. Calculating the Number of Moles of Copper (II) Phosphate in 129.4 g:
To find the number of moles of copper (II) phosphate in 129.4 g, we use the formula:
[
\text{moles} = \frac{\text{mass}}{\text{molar mass}}
]
Substitute the given values:
[
\text{moles} = \frac{129.4 \, \text{g}}{380.59 \, \text{g/mol}} \approx 0.340 \, \text{moles}
]
d. Calculating the Total Number of Atoms of Phosphorus:
Each molecule of copper (II) phosphate, Cu₃(PO₄)₂, contains 2 phosphorus (P) atoms. Therefore, for 0.340 moles of Cu₃(PO₄)₂, the number of moles of phosphorus atoms is:
[
\text{moles of phosphorus} = 0.340 \, \text{moles} \times 2 \, \text{atoms of phosphorus per formula unit} = 0.680 \, \text{moles of phosphorus}
]
To find the total number of atoms of phosphorus, multiply the moles of phosphorus by Avogadro’s number (( 6.022 \times 10^{23} ) atoms/mol):
[
\text{atoms of phosphorus} = 0.680 \, \text{moles} \times 6.022 \times 10^{23} \, \text{atoms/mol} \approx 4.09 \times 10^{23} \, \text{atoms}
]
Final Results:
- a. The formula for copper (II) phosphate is Cu₃(PO₄)₂.
- b. The molecular weight of copper (II) phosphate is 380.59 g/mol.
- c. The number of moles of copper (II) phosphate in 129.4 g is approximately 0.340 moles.
- d. The total number of atoms of phosphorus in 129.4 g of copper (II) phosphate is approximately 4.09 × 10²³ atoms.
These calculations provide an understanding of how to convert between grams, moles, and atoms for chemical compounds, particularly with ionic compounds like copper (II) phosphate.