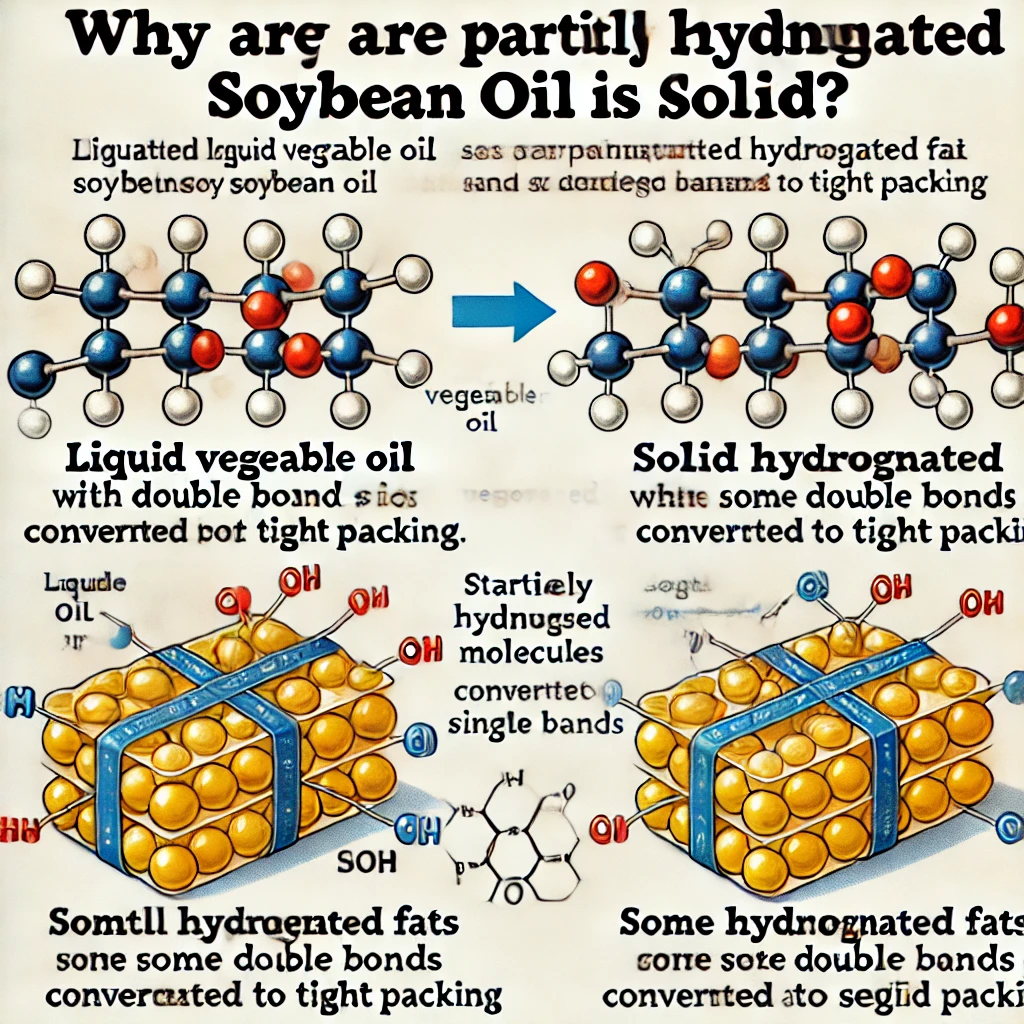
Margarine containing partially hydrogenated soybean oil is solid because
A). it contains only saturated fats.
B). it contains only trans fatty acids.
C). some of its double bonds have been converted to single bonds.
D). it contains only cis double bonds.
E). it contains only polyunsaturated fatty acids.
The correct answer and explanation is :
The correct answer is:
C) Some of its double bonds have been converted to single bonds.
Explanation:
Margarine is made from vegetable oils, which are naturally liquid at room temperature due to their high content of unsaturated fatty acids. These unsaturated fats have double bonds in their hydrocarbon chains, creating kinks that prevent them from packing closely together. As a result, vegetable oils remain in a liquid state.
To make margarine solid, manufacturers use partial hydrogenation, a process in which hydrogen gas is added to the oil in the presence of a metal catalyst (such as nickel). This process breaks some of the double bonds in the unsaturated fats, converting them into single bonds and making the fat more saturated. Since saturated fats have straight chains, they can pack more tightly together, leading to a more solid texture.
Why the Other Choices Are Incorrect:
- (A) It contains only saturated fats → Incorrect. Margarine is only partially hydrogenated, meaning it still contains some unsaturated fats. Fully hydrogenated oils would be completely solid and have a different texture.
- (B) It contains only trans fatty acids → Incorrect. While partial hydrogenation can create trans fats, margarine still contains some cis and saturated fats.
- (D) It contains only cis double bonds → Incorrect. The hydrogenation process can convert some cis double bonds to trans double bonds, altering the natural structure of the fatty acids.
- (E) It contains only polyunsaturated fatty acids → Incorrect. If margarine contained only polyunsaturated fats, it would remain liquid at room temperature instead of being semi-solid.
Thus, the correct answer is C, as the hydrogenation process removes some double bonds, making the fat more solid at room temperature.