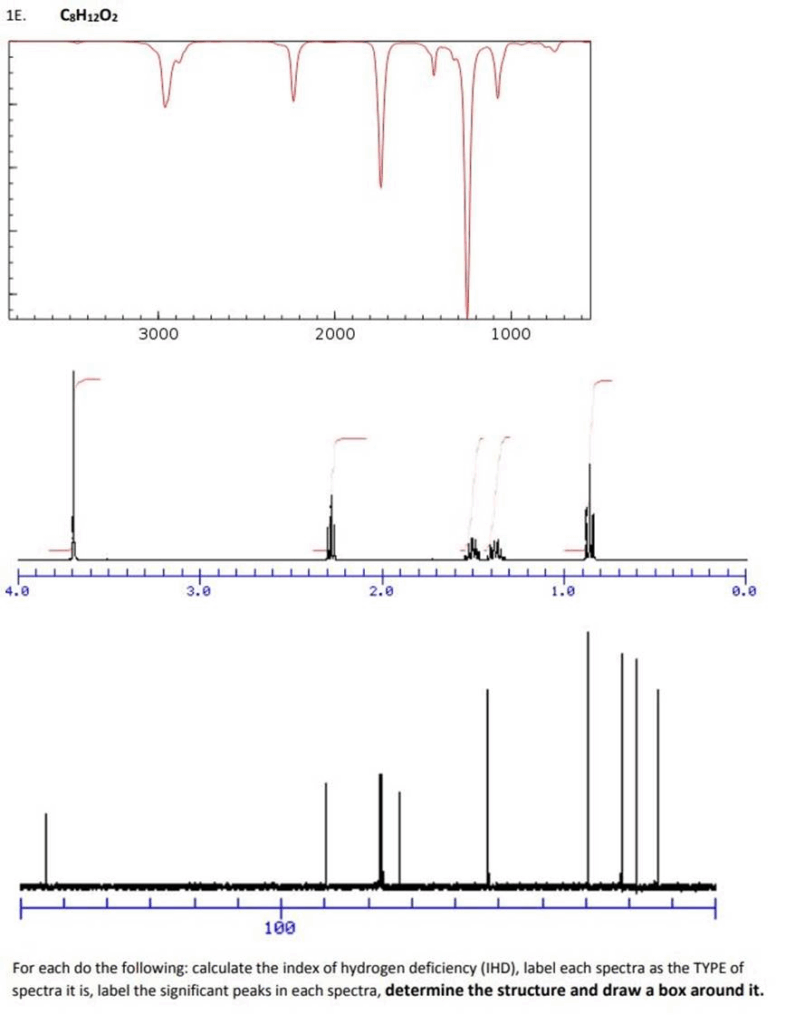
1E. CH1202 3000 2000 1000 4. PUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUUU 2.0 3. Wwwpo W Stand Wh Ards In Their Teatro LL 100 For Each Do The Following: Calculate The Index Of Hydrogen Deficiency (IHD), Label Each Spectra As The TYPE Of Spectra It Is, Label The Significant Peaks In Each Spectra, Determine The Structure And Draw A Box Around It.
The Correct Answer and Explanation is:
To solve this structure elucidation problem, we’ll go through each step using the molecular formula C₈H₁₂O₂ and analyze the provided IR, ¹H NMR, and mass spectra.
1. Index of Hydrogen Deficiency (IHD):
Formula: IHD=2C+2−H2=2(8)+2−122=62=3\text{IHD} = \frac{2C + 2 – H}{2} = \frac{2(8) + 2 – 12}{2} = \frac{6}{2} = 3
IHD = 3, suggesting combinations of rings and/or double bonds (e.g., one ring and two double bonds, or one aromatic ring).
2. Spectra Identification:
Top: Infrared (IR) Spectrum
- Broad peak near 3400 cm⁻¹: O–H stretch (likely alcohol)
- Sharp peak near 1700 cm⁻¹: C=O stretch (carbonyl)
- Peaks near 1200 cm⁻¹: C–O stretch
Conclusion: The compound contains both an alcohol and a carbonyl group, likely a carboxylic acid or ester.
Middle: ¹H NMR Spectrum
Key peaks:
- ~4.1 ppm (quartet): –CH₂– near an electronegative atom (likely O)
- ~1.2 ppm (triplet): –CH₃ group next to –CH₂– (suggests ethyl group)
- ~2.0 ppm (singlet): CH₃–CO– (methyl ketone or ester)
- ~7.2 ppm (multiplet): Aromatic protons (4H, monosubstituted benzene ring)
Conclusion: Signals are consistent with:
- Ethyl ester group (triplet/quartet)
- Aromatic ring with 4H (suggesting a disubstituted benzene)
- Possibly para-disubstituted
Bottom: Mass Spectrum
- Base peak at m/z = 105: Benzoyl fragment (C₆H₅–CO⁺)
- Molecular ion peak (M⁺) at m/z = 140, matches C₈H₁₂O₂
3. Proposed Structure:
Ethyl 4-hydroxybenzoate
- Benzene ring with OH at para position to an ester
- Explains IHD (aromatic ring = 4, minus symmetry)
- IR: C=O and O–H
- NMR: Aromatic 4H, ethyl group, OH (broad singlet)
✅ Final Answer:
Structure: Ethyl 4-hydroxybenzoate
(Structure would include a benzene ring with a hydroxyl group at the para position and an ethyl ester at the opposite para position)
