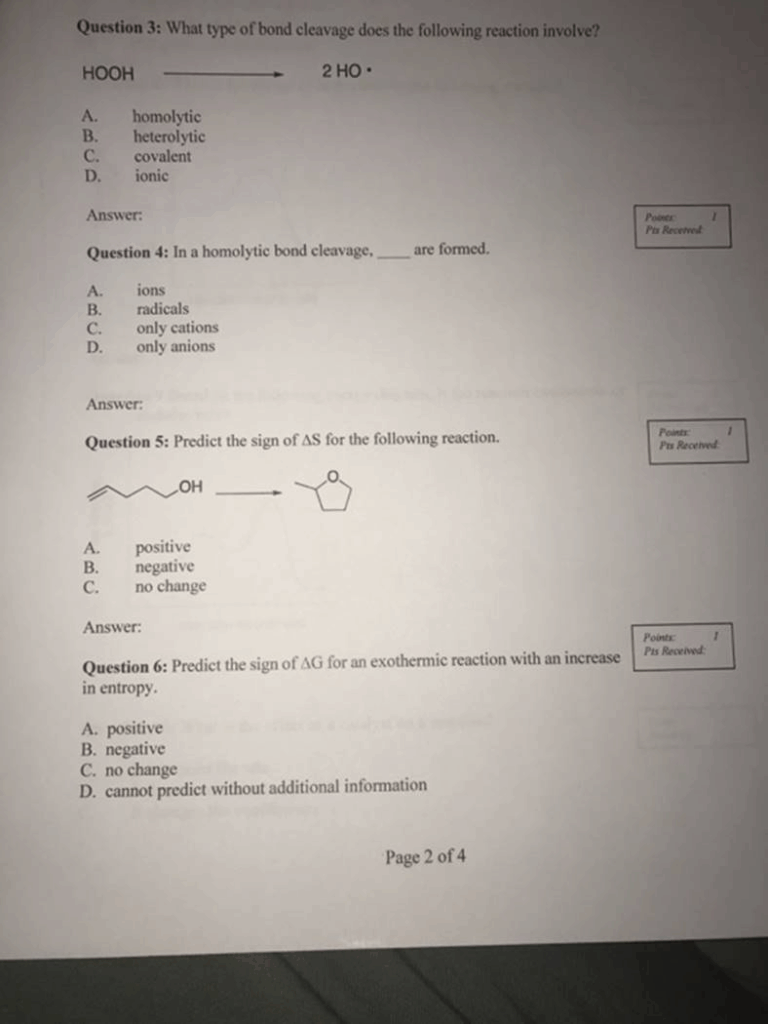
Question 3: What type of bond cleavage does the following reaction involve? HOOH 2 HO A. homolytic B. heterolytic C. covalent D. ionic Answer Points Pts Received Question 4: In a homolytic bond cleavage, are formed. A. ions B. radicals C. only cations D. only anions Answer Question 5: Predict the sign of AS for the following reaction. Points Pts Received A. positive B. negative C. no change Answer: Points Pts Recened Question 6: Predict the sign of AG for an exothermic reaction with an increase in entropy. A. positive B. negative C. no change D. cannot predict without additional information Page 2 of 4
The Correct Answer and Explanation is:
Question 3: What type of bond cleavage does the following reaction involve?
HOOH → 2 HO•
Answer: A. homolytic
Question 4: In a homolytic bond cleavage, ___ are formed.
Answer: B. radicals
Question 5: Predict the sign of ΔS (entropy change) for the following reaction:
HOOH → 2 HO•
Answer: A. positive
Question 6: Predict the sign of ΔG (Gibbs free energy change) for an exothermic reaction with an increase in entropy.
Answer: B. negative
Explanation
In chemistry, bond cleavage refers to the breaking of a chemical bond. There are two main types: homolytic and heterolytic. In homolytic cleavage, the bond breaks evenly, and each atom retains one of the shared electrons, producing radicals, which are species with unpaired electrons. In contrast, heterolytic cleavage results in the formation of ions, where one atom retains both bonding electrons.
For Question 3, the reaction shows hydrogen peroxide (HOOH) breaking into two hydroxyl radicals (HO•), each with an unpaired electron. This clearly indicates homolytic cleavage. Therefore, the correct answer is A. homolytic.
Question 4 follows directly from this concept—homolytic cleavage produces radicals, not ions. Hence, the answer is B. radicals.
In Question 5, the reaction converts one molecule (HOOH) into two particles (HO•), increasing the number of molecules and disorder. This leads to an increase in entropy (ΔS > 0). So the sign of ΔS is positive.
Question 6 deals with Gibbs free energy change (ΔG), which determines the spontaneity of a reaction. The formula is: ΔG=ΔH−TΔS\Delta G = \Delta H – T\Delta S
For an exothermic reaction (ΔH < 0) with an increase in entropy (ΔS > 0), both terms favor a negative ΔG, which means the reaction is spontaneous. Therefore, the correct answer is B. negative.
Understanding these fundamental thermodynamic and chemical concepts is essential for predicting reaction mechanisms and behaviors in chemical systems.
