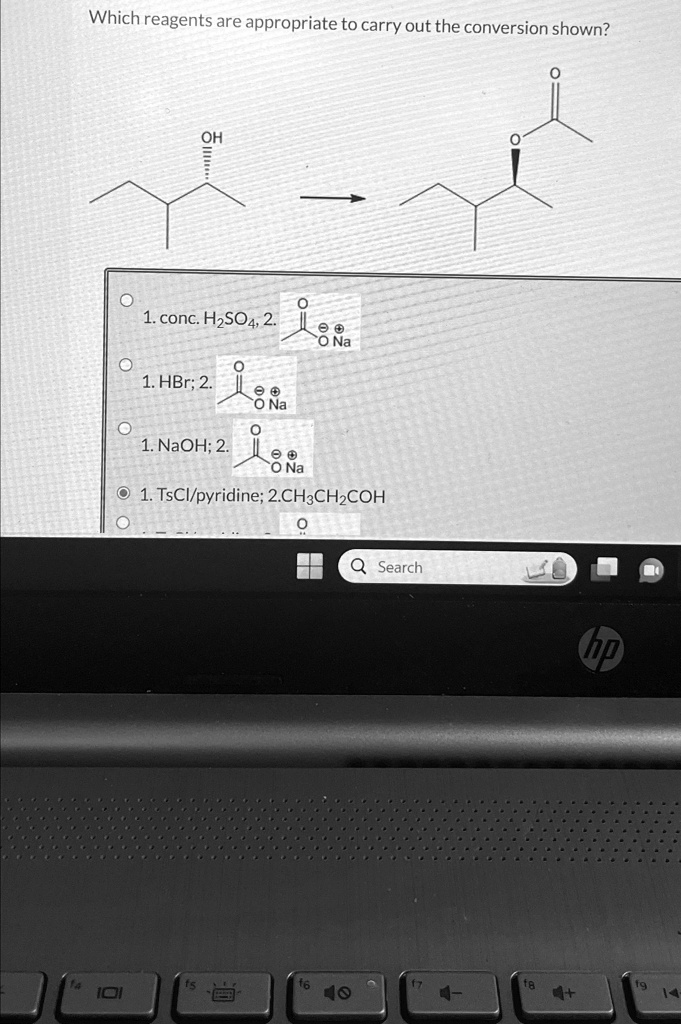
Which reagents are appropriate to carry out the conversion shown? o conc. H_(2)SO_(4),2. HBr;2. NaOH;2. TsC(l)/() pyridine; 2.CH_(3)CH_(2)COH Search Which reagents are appropriate to carry out the conversion shown? OH 1.conc.HSO4,2 0 O.Na 1.HBr2 00 ONa 1.NaOH;2. ONa O1.TsCI/pyridine;2.CH3CHCOH Search I0 40
The Correct Answer and Explanation is:
Correct Answer:
1. TsCl/pyridine; 2. CH₃CH₂CO⁻ (ethoxide in ethanol)
Explanation
The transformation shown is a substitution reaction, where the hydroxyl group (–OH) in the starting compound is replaced by a propionate group (–O–COCH₂CH₃), resulting in an ester. Specifically, we are converting a secondary alcohol into an ester using a two-step mechanism.
Step-by-Step Mechanism:
Step 1: Tosylation using TsCl/Pyridine
- TsCl (tosyl chloride) reacts with the alcohol in the presence of pyridine (a base) to form a tosylate ester (R–OTs).
- Tosylates are excellent leaving groups, much better than –OH.
- The reaction mechanism involves activation of the hydroxyl group, converting it into a better leaving group without disturbing the stereochemistry of the carbon center.
Step 2: Nucleophilic Substitution using CH₃CH₂CO⁻
- The ethyl carboxylate anion (ethoxide from propionic acid) acts as a nucleophile.
- It displaces the tosyl group via an SN2 reaction, forming an ester.
- Since this is an SN2 reaction on a secondary carbon, the inversion of configuration might be considered, but tosylate formation maintains the configuration until the backside attack by the nucleophile, resulting in overall inversion of stereochemistry. However, the stereochemistry is not the focus here—formation of the ester is.
Why Other Options Are Incorrect:
- conc. H₂SO₄: Acidic conditions typically lead to dehydration or ether formation, not esterification with a carboxylate nucleophile.
- HBr: This would lead to substitution with Br⁻, not ester formation.
- NaOH: Would not activate –OH to become a good leaving group or promote ester formation.
Conclusion:
The best method is tosylation followed by nucleophilic attack with a carboxylate anion, which is achieved by TsCl/pyridine; then CH₃CH₂CO⁻. This efficiently forms the ester shown in the product.
