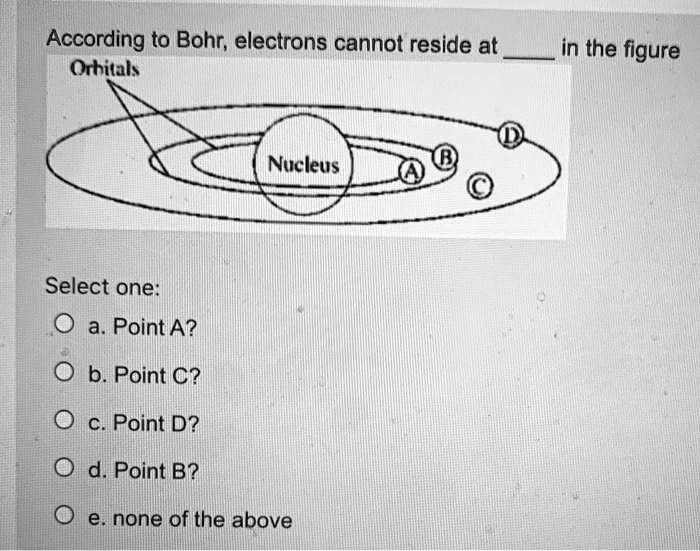
According to Bohr; electrons cannot reside at Orhitals in the figure Nucleus Select one: a. Point A? b. Point C? Point D? d. Point B? none of the above
The Correct Answer and Explanation is:
The correct answer is: b. Point C
Explanation:
According to Niels Bohr’s atomic model, electrons revolve around the nucleus in fixed paths or quantized energy levels called orbitals or shells. These orbits correspond to specific energy levels, and an electron in an atom is allowed to occupy only these specific orbits.
Here are the key principles of Bohr’s model relevant to the question:
- Electrons can only exist in specific orbits (energy levels), not in between.
- Energy is quantized, meaning electrons must absorb or emit a fixed amount of energy to move between levels.
- Electrons cannot reside between these energy levels—they “jump” instantly from one level to another.
In the provided image:
- Points A, B, and D are located directly on the defined circular orbitals.
- Point C, however, lies between two orbitals, which is not allowed in the Bohr model.
This is why electrons cannot reside at Point C. The Bohr model restricts their position to fixed orbits—any position that falls between these orbits (like Point C) is forbidden.
Additional Context:
Bohr’s model was an early step in the development of quantum theory. While it’s been replaced by more accurate quantum mechanical models (which use probability clouds instead of fixed orbits), Bohr’s model still provides a foundational way to understand atomic structure in introductory physics and chemistry.
To summarize, Point C is not a valid location for an electron in the Bohr model because it’s not on a defined energy level. Hence, the correct answer is b. Point C.
