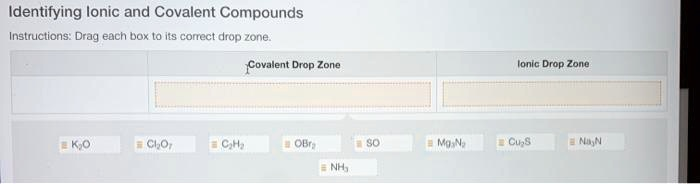
Identifying Ionic and Covalent Compounds Instructions: Drag each box to its correct drop zone: ~Covalent Drop Zone Ionic Drop Zone Cl2 CH4 O2 Mg3N2 Cu3P2 NH3
The Correct Answer and Explanation is:
To correctly classify the compounds into ionic and covalent, we need to understand the nature of chemical bonding:
- Ionic bonds form between metals and nonmetals, where electrons are transferred.
- Covalent bonds form between nonmetals, where electrons are shared.
Correct Classification:
Covalent Compounds:
- Cl₂O₇ – Both chlorine and oxygen are nonmetals → covalent.
- C₂H₂ – Carbon and hydrogen are nonmetals → covalent.
- OBr₂ – Oxygen and bromine are nonmetals → covalent.
- SO – Sulfur and oxygen are nonmetals → covalent.
- NH₃ – Nitrogen and hydrogen are nonmetals → covalent.
Ionic Compounds:
- K₂O – Potassium (metal) and oxygen (nonmetal) → ionic.
- Mg₃N₂ – Magnesium (metal) and nitrogen (nonmetal) → ionic.
- Cu₃S – Copper (metal) and sulfur (nonmetal) → ionic.
- Na₃N – Sodium (metal) and nitrogen (nonmetal) → ionic.
Explanation
Chemical compounds can be broadly categorized based on the type of bonding that holds their atoms together. These bonds are primarily ionic or covalent. Understanding how to distinguish between them is crucial in chemistry.
Ionic compounds are formed when one or more electrons are transferred from a metal to a nonmetal. This transfer creates ions: positively charged cations (metals) and negatively charged anions (nonmetals). The electrostatic attraction between these oppositely charged ions forms a strong ionic bond. For example, K₂O (potassium oxide) is ionic because potassium donates electrons to oxygen. Similarly, Mg₃N₂, Cu₃S, and Na₃N involve metals bonding with nonmetals, resulting in ionic compounds.
In contrast, covalent compounds form when atoms, typically nonmetals, share electrons to achieve stability. These shared electrons create a bond between the atoms. Covalent compounds usually have lower melting and boiling points compared to ionic ones and may exist as gases, liquids, or solids. Examples include Cl₂O₇ (dichlorine heptoxide), C₂H₂ (ethyne), OBr₂ (oxygen dibromide), SO (sulfur monoxide), and NH₃ (ammonia). These are all composed of nonmetals, and their atoms are held together by shared electron pairs.
By identifying whether elements in a compound are metals or nonmetals, and understanding how they bond, we can accurately classify compounds as ionic or covalent.
