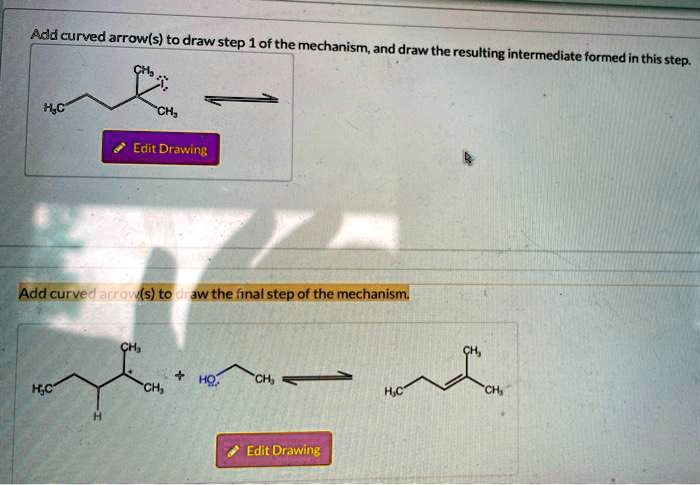
Add curved arrows to draw step 1 of the mechanism; and draw the resulting intermediate formed in this step. ‘CH; Edit Drawing Add curved arrows to draw the final step of the mechanism. H
The Correct Answer and Explanation is:
Answer and Explanation
This image shows a question about a reaction mechanism involving a carbocation intermediate and curved arrow formalism. It appears to be an acid-catalyzed reaction, most likely acid-catalyzed dehydration of an alcohol or acid-catalyzed rearrangement of a carbocation.
Step 1: Protonation of the Alcohol and Loss of Water
In the top reaction box, we are starting with:
2-methyl-2-butanol:
CH3
|
CH3-C-CH2CH3
|
OH
This is a tertiary alcohol. The mechanism begins with:
- Protonation of the hydroxyl group (-OH) by an acid (H₃O⁺ or H⁺).
- The protonated -OH (now -OH₂⁺) becomes a good leaving group.
- It leaves, forming a tertiary carbocation.
Curved arrows to draw in Step 1:
- From the lone pair on oxygen of the -OH group to a proton (H⁺).
- From the bond between oxygen and the protonated water (-OH₂⁺) to oxygen, showing the loss of water (H₂O).
Intermediate formed:
A tertiary carbocation:
CH3
|
CH3-C⁺-CH2CH3
This carbocation is stabilized due to hyperconjugation and inductive effects from the three alkyl groups.
Final Step: Nucleophilic Attack and Product Formation
From the lower part of the image:
We have the carbocation reacting with water:
- Water (H₂O) acts as a nucleophile, attacking the positively charged carbon.
- A proton transfer occurs after the attack, yielding the final alcohol product.
Curved arrows to draw in the final step:
- From a lone pair on water to the carbocation center.
- From the O-H bond of the newly added water group to oxygen (deprotonation step).
This forms:
2-butanol (after rearrangement and deprotonation):
CH3CH(OH)CH2CH3
Summary
This mechanism showcases the acid-catalyzed substitution involving a carbocation intermediate. In step 1, the hydroxyl group of 2-methyl-2-butanol is protonated by an acid (not shown explicitly, but implied). Protonation converts the -OH into a better leaving group (-OH₂⁺), which then departs, generating a stable tertiary carbocation at the 2-position. This is the rate-determining step and key in carbocation-based reactions such as SN1 and E1.
The intermediate formed here is 2-methyl-2-butyl carbocation, which is stabilized by hyperconjugation and inductive effects from its surrounding alkyl groups. This stability helps the reaction proceed efficiently.
In the final step, a water molecule acts as a nucleophile, attacking the carbocation center. This forms an oxonium ion intermediate (protonated alcohol). Finally, a base (often water itself) abstracts a proton from the oxonium ion, yielding the neutral alcohol product.
The overall mechanism illustrates how protonation, carbocation stability, and nucleophilic attack work together in acid-catalyzed reactions. Curved arrows are crucial to show electron movement: lone pair to bond (nucleophilic attack), and bond to atom (leaving group or deprotonation). This type of mechanism is commonly seen in organic transformations such as alcohol dehydration or substitution under acidic conditions.
