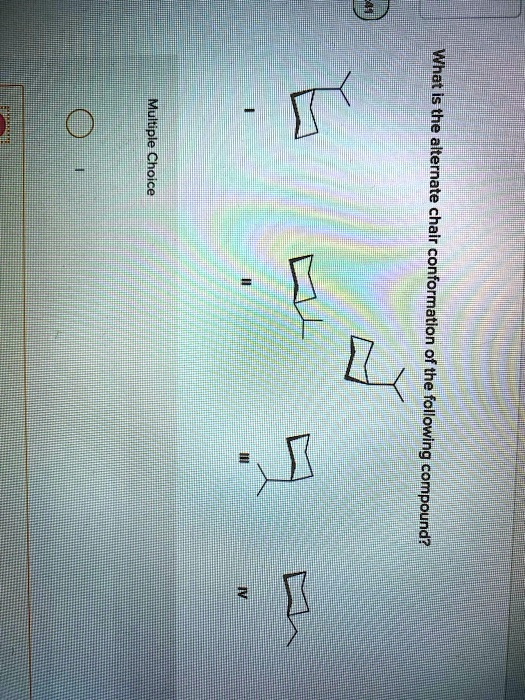
What is the alternate chair conformation of the following compound? Multiple Cyclohexane
The Correct Answer and Explanation is:
Correct Answer: II
Explanation
The question is asking for the alternate chair conformation of a substituted cyclohexane ring. In this specific case, we’re shown a chair conformation of methylcyclohexane where the methyl group is in the axial position in the initial structure (Option I).
In cyclohexane, the most stable conformation is the chair conformation because it minimizes torsional strain. Cyclohexane undergoes a chair flip (ring flip), a process that converts one chair form into another. During a ring flip:
- Axial groups become equatorial.
- Equatorial groups become axial.
- The relative cis/trans stereochemistry remains the same.
For the given molecule (structure I), the methyl group is in the axial position. Upon a chair flip:
- The methyl group will move from the axial position to the equatorial position on the opposite side of the ring.
- All hydrogens switch accordingly, maintaining the stereochemistry.
The most stable conformation for methylcyclohexane is the one where the bulky methyl group occupies the equatorial position, because this reduces 1,3-diaxial interactions and steric hindrance.
Now let’s analyze the choices:
- Option I shows the original conformation with the methyl group axial.
- Option II shows the methyl group in the equatorial position on the same carbon, which matches the expected alternate conformation after a chair flip.
- Option III also has the methyl group axial but on a different carbon—this is not the correct flip.
- Option IV shows a different substitution pattern entirely.
Thus, Option II correctly shows the alternate chair conformation, with the methyl group now in the equatorial position.
This alternate chair conformation (II) is more stable than the original (I), which has the methyl group in the axial position. Therefore, option II is both the correct alternate conformation and the more energetically favorable form.
