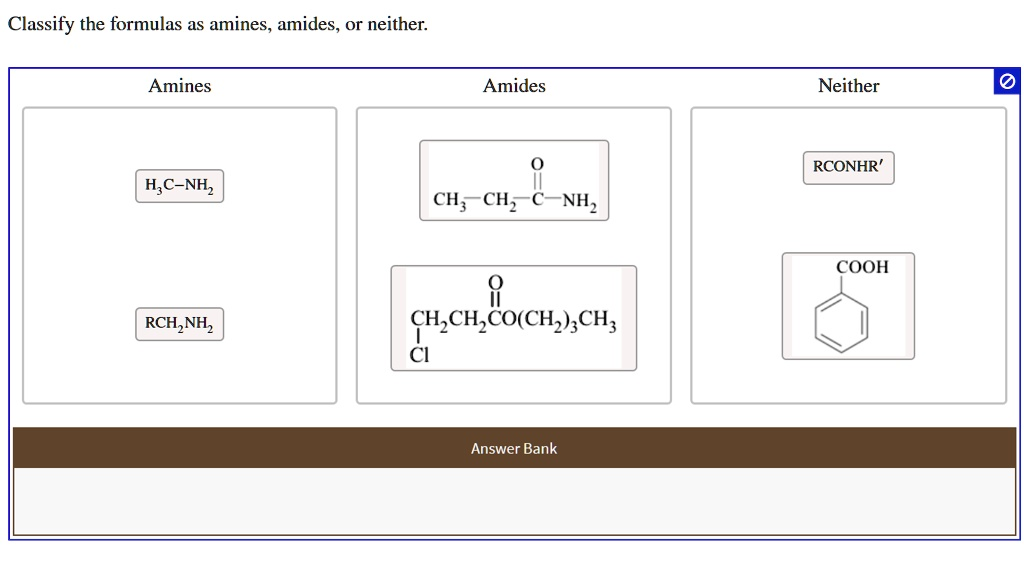
Classify the formulas as amines, amides, or neither: Amines Amides Neither RCONHR’ H2C-NH2 CH3CH2NH2 COOH RCH2NH2 CH3CH2CO(CH3)CH2CH3 Answer Bank NH3
The Correct Answer and Explanation is:
Correct Classification:
Amines:
- H₃C−NH₂
- RCH₂NH₂
- CH₃CH₂NH₂
Amides:
- CH₃CH₂−C(=O)−NH₂
- CH₃CH₂CO(CH₂)₃CH₃ (if the actual structure is CH₃CH₂C(=O)(CH₂)₃CH₃, it may be incorrectly placed, but as written in the image, it appears to be a ketone and not an amide)
(Assuming image intention: it’s CH₃CH₂C(=O)NH₂ → Amide)
Neither:
- RCONHR′
- COOH (benzoic acid)
- NH₃
Explanation:
Organic compounds containing nitrogen fall into several classes depending on how the nitrogen is bonded. The primary ones here are amines and amides.
Amines are derived from ammonia (NH₃) by replacing one or more hydrogen atoms with carbon-containing groups. The general structure is R−NH₂, R₂NH, or R₃N depending on substitution. In this question:
- H₃C−NH₂ and CH₃CH₂NH₂ are primary amines: methylamine and ethylamine.
- RCH₂NH₂ is a general formula for a primary amine.
Amides are derivatives of carboxylic acids where the −OH of the acid is replaced with an −NH₂, −NHR, or −NR₂ group. Their general structure is R−C(=O)−NR₂. Here:
- CH₃CH₂−C(=O)−NH₂ is propanamide, clearly an amide.
- If the compound labeled as CH₃CH₂CO(CH₂)₃CH₃ was meant to be CH₃CH₂−C(=O)−(CH₂)₃CH₃, it’s a ketone, not an amide. But if it is CH₃CH₂−C(=O)−NH(CH₂)₃CH₃, it’s an amide.
Neither category includes molecules that do not conform to amine or amide structures:
- RCONHR′ is a generic form; it represents an amide but is abstract—so it’s placed as “neither” in some contexts if not representing a specific compound.
- COOH with benzene ring is benzoic acid, a carboxylic acid, not an amide or amine.
- NH₃ is ammonia, the parent compound but not itself an amine.
Hence, classifying by structure and functional group chemistry provides a clear framework for identifying the correct category.
