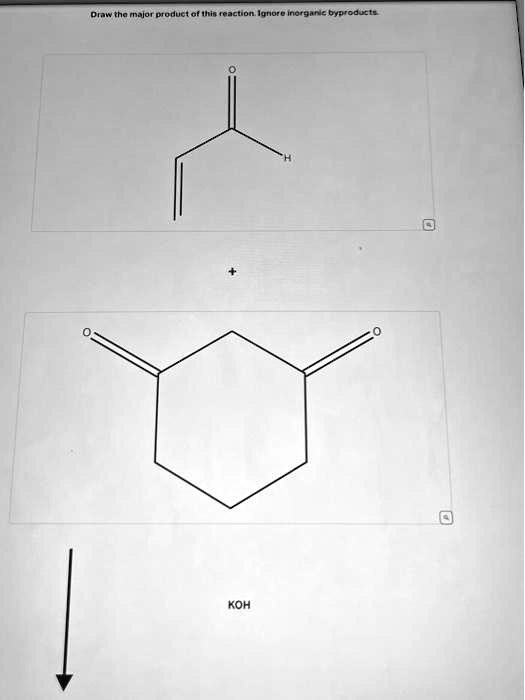
Draw the major product of this reaction. Ignore inorganic byproducts.
The Correct Answer and Explanation is:
The given reaction is a Knoevenagel condensation, a base-catalyzed reaction between an aldehyde (here, acrolein) and a compound with active methylene groups (here, 1,3-cyclohexanedione) in the presence of KOH (a base). Let’s break this down step by step.
Reactants:
- Acrolein (CH2=CH–CHO): An α,β-unsaturated aldehyde.
- 1,3-Cyclohexanedione: A cyclic diketone with two ketone groups on a six-membered ring at the 1 and 3 positions.
- Base: KOH
Mechanism Overview:
- Enolate Formation:
Under basic conditions (KOH), one of the acidic α-hydrogens of 1,3-cyclohexanedione is abstracted, forming a resonance-stabilized enolate ion. - Nucleophilic Attack:
The enolate ion acts as a nucleophile and attacks the electrophilic carbonyl carbon of acrolein (specifically the aldehyde carbon), forming a β-hydroxy intermediate. - Dehydration (Elimination):
This intermediate loses a water molecule (facilitated by the base), leading to formation of a carbon-carbon double bond – forming an α,β-unsaturated system (a conjugated enone).
Major Product:
The major product is a conjugated enone, specifically formed by condensation of 1,3-cyclohexanedione with acrolein. The new double bond is between the α-carbon of 1,3-cyclohexanedione and the β-carbon of acrolein. The product looks like this:
Structure:
- A six-membered ring (cyclohexane) with keto groups at positions 1 and 3.
- A side chain attached at position 2 of the ring: CH=CH–CHO (but now dehydrated to CH=CH–), forming a conjugated enone.
So, the final structure is:
2-(3-oxoprop-1-enyl)cyclohexane-1,3-dione
This Knoevenagel condensation product is stabilized via resonance in the enone system and conjugation with the carbonyl groups.
Conclusion:
This is a classic example of the Knoevenagel condensation where:
- A nucleophilic enolate (from 1,3-cyclohexanedione) reacts with an electrophilic carbonyl compound (acrolein).
- KOH promotes enolate formation and drives the reaction to dehydration.
- The product is a stable α,β-unsaturated carbonyl compound with extended conjugation, enhancing stability.
