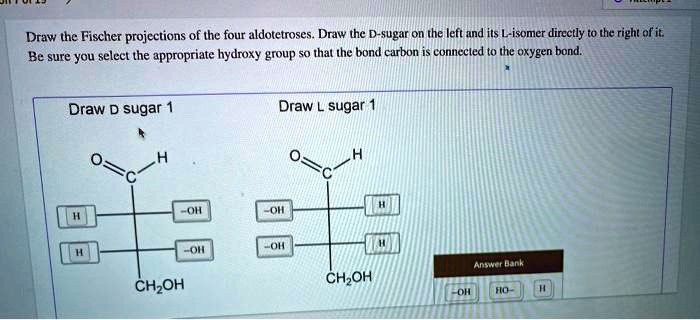
Draw the Fischer projections of the four aldotetroses. Draw the D-sugar on the left and its L-isomer directly to the right of it. Be sure you select the appropriate hydroxy group so that the carbon bonded to the oxygen is connected to it. Draw D-sugar Draw sugar CH2OH CH2OH
The Correct Answer and Explanation is:
The question asks you to draw the Fischer projections of the four aldotetroses — specifically, the D- and L-isomers of one aldotetrose. Aldotetroses are sugars with four carbon atoms and an aldehyde group at the top. There are four stereoisomers (two pairs of D- and L-enantiomers), but this question focuses on just one pair.
From the image, we see the Fischer projections for D-erythrose on the left and L-erythrose on the right.
Correct Structures:
D-erythrose (D-sugar 1):
mathematicaCopyEditCHO
|
H–C–OH
|
H–C–OH
|
CH2OH
L-erythrose (L-sugar 1):
mathematicaCopyEditCHO
|
HO–C–H
|
HO–C–H
|
CH2OH
These are correctly drawn in the image. The distinguishing feature between D- and L- isomers lies in the orientation of the hydroxyl group on the highest-numbered chiral carbon (carbon 3 in aldotetroses). In D-sugars, the hydroxyl on the bottom chiral center points to the right, and in L-sugars, it points to the left.
Explanation
Aldotetroses are monosaccharides composed of four carbon atoms and an aldehyde functional group. The general structure includes an aldehyde at carbon 1 and hydroxyl groups at the other chiral centers. They have two chiral centers (C2 and C3), meaning they can form 2² = 4 stereoisomers. These stereoisomers are divided into two sets of enantiomers (mirror images): erythrose and threose, each with D- and L-forms.
In a Fischer projection:
- The most oxidized carbon (the aldehyde, CHO) is placed at the top.
- Horizontal lines represent bonds coming out of the plane toward the viewer.
- Vertical lines represent bonds going into the plane.
For D-aldoses, the hydroxyl group on the last chiral center (farthest from the aldehyde) is on the right.
For L-aldoses, it’s on the left.
The image correctly displays D-erythrose and its mirror image L-erythrose:
- Both have hydroxyl groups on the same side for C2 and C3 (right for D, left for L).
- This defines them as the “erythro” pair, where the -OH groups are on the same side.
Thus, the image is accurate for one of the four aldotetroses. The other two (threose and its mirror) would have the hydroxyls on opposite sides at the chiral centers.
This fundamental understanding is crucial in stereochemistry and carbohydrate biochemistry, where the configuration determines biochemical reactivity and biological function.
