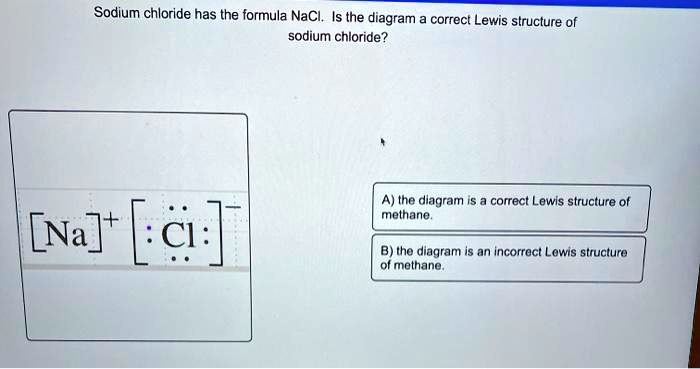
Sodium chloride has the formula NaCl. Is the diagram a correct Lewis structure of sodium chloride? [Na]
[:Cl:]
A) the diagram is a correct Lewis structure of methane. B) the diagram is an incorrect Lewis structure of methane.
The correct answer is: Neither A nor B are correct options for this question.
However, if forced to choose from the given options, both are irrelevant because the question is about sodium chloride (NaCl), but both answer choices mention methane, which is unrelated.
✅ Correct Interpretation:
The image shows a Lewis structure of sodium chloride (NaCl). In the diagram:
- Sodium (Na) is shown as Na⁺, indicating it has lost one electron.
- Chlorine (Cl) is shown as Cl⁻ with a full octet (8 valence electrons), indicating it has gained one electron.
- The square brackets and charges represent ionic bonding, not covalent bonding.
This is the correct way to represent the Lewis structure of an ionic compound like NaCl. Sodium donates its one valence electron to chlorine, resulting in:
- A Na⁺ cation (no valence electrons left),
- A Cl⁻ anion (with a full octet),
- And an electrostatic attraction between them forms the ionic bond.
❌ Why the Answer Choices Are Invalid:
- Choice A: “The diagram is a correct Lewis structure of methane.”
This is incorrect. Methane is CH₄, a covalent molecule where one carbon shares electrons with four hydrogen atoms. It has nothing to do with NaCl. - Choice B: “The diagram is an incorrect Lewis structure of methane.”
Again, this is irrelevant. The question is about sodium chloride, not methane.
🧠 Conclusion:
- The diagram correctly shows the Lewis structure of sodium chloride (NaCl) as an ionic compound.
- The given answer options are not applicable to the question.
- This appears to be a question/answer mismatch error — possibly a typo or programming bug in the multiple-choice options.
