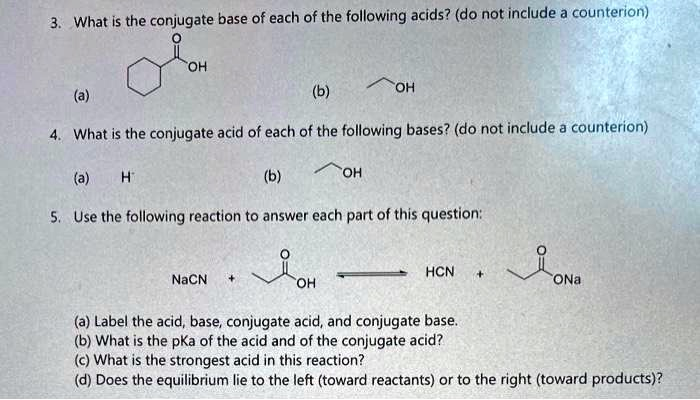
What is the conjugate base of each of the following acids? (do not include a counterion) ???? OH (a) (b) OH 4. What is the conjugate acid of each of the following bases? (do not include a counterion) (a) H (b) OH 5. Use the following reaction to answer each part of this question: NaCN + OH HCN + JoNa (a) Label the acid, base, conjugate acid, and conjugate base. (b) What is the pka of the acid and of the conjugate acid? (c) What is the strongest acid in this reaction? (d) Does the equilibrium lie to the left (toward reactants) or to the right (toward products)?
The Correct Answer and Explanation is:
3. What is the conjugate base of each of the following acids?
(a) ![Structure] A carboxylic acid (likely phenylacetic acid):
- Answer: The conjugate base is the carboxylate anion: Ph-CH2-COO−\text{Ph-CH}_2\text{-COO}^-Ph-CH2-COO− (Deprotonation of the –COOH group)
(b) Ethanol (CH₃CH₂OH):
- Answer: The conjugate base is the ethoxide ion: CH3CH2O−\text{CH}_3\text{CH}_2\text{O}^-CH3CH2O−
4. What is the conjugate acid of each of the following bases?
(a) H⁻ (hydride):
- Answer: The conjugate acid is H₂ (hydrogen gas): H−+H+→H2\text{H}^- + \text{H}^+ \rightarrow \text{H}_2H−+H+→H2
(b) CH₃CH₂O⁻ (ethoxide ion):
- Answer: The conjugate acid is ethanol (CH₃CH₂OH)
5. Reaction:
NaCN+CH3CH2COOH⇌HCN+CH3CH2COO−\text{NaCN} + \text{CH}_3\text{CH}_2\text{COOH} \rightleftharpoons \text{HCN} + \text{CH}_3\text{CH}_2\text{COO}^-NaCN+CH3CH2COOH⇌HCN+CH3CH2COO−
(a) Label each:
- Acid: CH₃CH₂COOH (propionic acid)
- Base: CN⁻ (from NaCN)
- Conjugate acid: HCN
- Conjugate base: CH₃CH₂COO⁻
(b) pKa values:
- CH₃CH₂COOH (propionic acid): ~4.9
- HCN: ~9.2
(c) Strongest acid:
- CH₃CH₂COOH (lower pKa = stronger acid)
(d) Equilibrium direction:
- Equilibrium favors the formation of the weaker acid and base.
- Since CH₃CH₂COOH is a stronger acid than HCN, the reaction favors the right (products).
Explanation
In acid-base chemistry, every acid has a conjugate base, formed by removing a proton (H⁺), and every base has a conjugate acid, formed by adding a proton. For instance, phenylacetic acid loses H⁺ to form its carboxylate conjugate base, while ethanol forms ethoxide ion when deprotonated.
Conversely, hydride (H⁻) accepts a proton to form diatomic hydrogen gas (H₂), and the ethoxide ion gains a proton to regenerate ethanol, showing the reversibility of acid-base reactions.
In the reaction between NaCN and propionic acid (CH₃CH₂COOH), we identify propionic acid as the acid (donates H⁺) and cyanide ion (CN⁻) as the base (accepts H⁺). Their conjugate counterparts are HCN (from CN⁻) and propionate (CH₃CH₂COO⁻, from the acid).
The strength of acids is compared using their pKa values. A lower pKa indicates a stronger acid. Here, propionic acid has a pKa of ~4.9, while HCN has a pKa of ~9.2, meaning propionic acid is stronger. Since reactions typically favor the formation of the weaker acid and base (equilibrium principle), the reaction shifts toward the right side — favoring HCN and the propionate ion.
This understanding is foundational in predicting acid-base reaction direction, strength comparisons, and ion stability — key skills in general and organic chemistry.
