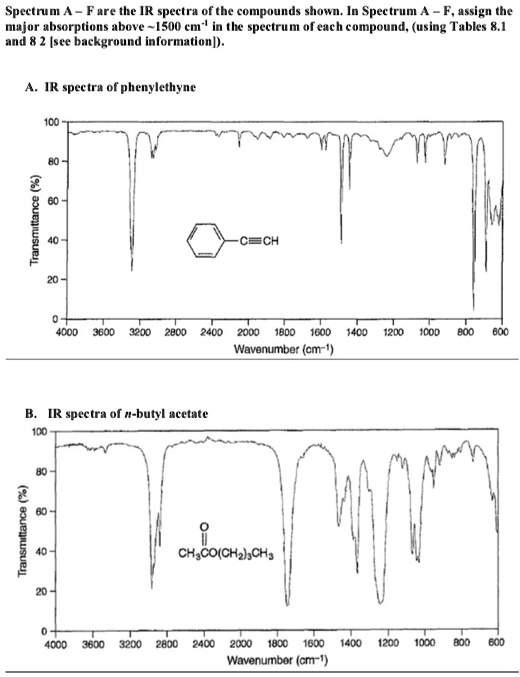
Spectrum A — F are the IR spectra of the compounds shown. In Spectrum A — F, assign the major absorptions above ~1500 cm
in the spectrum of each compound, (using Tables 8.1 and 8.2 [see background information]). A. IR spectra of phenylethyne 100 Transmittance (%) 80 60 40 20 0 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 600 Wavenumber (cm
) B. IR spectra of n-butyl acetate 100 Transmittance (%) 80 60 40 20 0 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 600 Wavenumber (cm
)
The Correct Answer and Explanation is:
Let’s analyze and assign the major IR absorptions above ~1500 cm⁻¹ for the two given spectra:
A. IR Spectrum of Phenylethyne
Structure: Phenylethyne (C₆H₅–C≡CH) is an aromatic alkyne.
Major IR Absorptions:
- ~3300 cm⁻¹ – Sharp, strong ≡C–H stretch (sp-hybridized C–H bond typical of terminal alkynes).
- ~2100–2200 cm⁻¹ – Weak C≡C stretch (typical alkyne triple bond stretching).
- ~3030 cm⁻¹ – Aromatic C–H stretch (sp² hybridized C–H).
- ~1600 and ~1500 cm⁻¹ – Aromatic C=C stretching (conjugated ring modes).
These peaks confirm the presence of both an aromatic ring and a terminal alkyne.
B. IR Spectrum of n-Butyl Acetate
Structure: CH₃COO(CH₂)₃CH₃ – an ester with an aliphatic chain.
Major IR Absorptions:
- ~1740 cm⁻¹ – Strong, sharp C=O (carbonyl) stretch (typical for esters).
- ~2950–2850 cm⁻¹ – C–H stretches (sp³ C–H from aliphatic chains).
- ~1240–1050 cm⁻¹ – Strong, complex C–O stretch (asymmetric/symmetric ester vibrations).
- ~1450 and ~1370 cm⁻¹ – C–H bending from –CH₂ and –CH₃ groups.
Explanation (300 words)
Infrared (IR) spectroscopy is a powerful technique used to identify functional groups within a molecule based on the absorption of infrared radiation, which causes molecular vibrations. Each functional group absorbs IR radiation at a characteristic wavenumber, typically measured in cm⁻¹.
In Spectrum A, the IR spectrum of phenylethyne, several key absorptions are noted. The sharp absorption near 3300 cm⁻¹ indicates a terminal alkyne C–H stretch (sp hybridized). The weak absorption near 2100–2200 cm⁻¹ is consistent with a carbon–carbon triple bond (C≡C) stretch. Additionally, aromatic C–H stretching appears near 3030 cm⁻¹, and characteristic C=C stretches of the benzene ring occur near 1600 cm⁻¹ and 1500 cm⁻¹, confirming the presence of an aromatic ring.
In Spectrum B, the IR spectrum of n-butyl acetate, the strongest absorption appears at ~1740 cm⁻¹, which corresponds to the C=O stretching vibration of the ester functional group. The peaks between 2950–2850 cm⁻¹ are due to aliphatic C–H stretching from the butyl chain. The ester also exhibits C–O stretching vibrations in the 1240–1050 cm⁻¹ range. Additionally, C–H bending modes near 1450 and 1370 cm⁻¹ further support the presence of methyl and methylene groups.
By comparing IR absorption peaks to known reference values (Tables 8.1 and 8.2), the functional groups in both compounds are clearly identified. This makes IR spectroscopy an essential tool for structural determination in organic chemistry.
