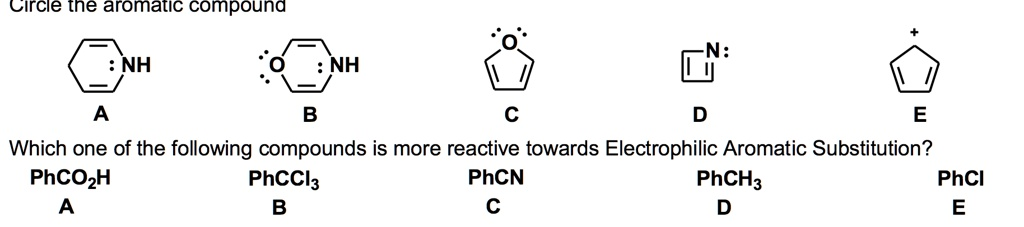
Circle In the aromatic compound NH NH Which one of the following compounds is more reactive towards Electrophilic Aromatic Substitution? PhCO2H PhCCl3 PhCN PhCH3 PhCl
The Correct Answer and Explanation is:
Here are the correct answers and explanations for the two questions.
Question 1: Circle the aromatic compound
Correct Answer: C
Explanation:
A compound is considered aromatic if it adheres to Hückel’s criteria for aromaticity:
- It must be cyclic.
- It must be planar.
- It must be fully conjugated, meaning every atom in the ring must have an available p-orbital.
- It must satisfy Hückel’s rule, containing (4n + 2) π electrons, where ‘n’ is a non-negative integer (0, 1, 2, etc.).
Let’s analyze the given options:
- A and B: These six-membered rings are not fully conjugated. They contain sp³ hybridized carbon atoms that break the continuous overlap of p-orbitals around the ring. Therefore, they are not aromatic.
- C (Furan): This compound is cyclic and planar. It is fully conjugated, as the oxygen atom has a lone pair of electrons in a p-orbital that participates in the ring’s π system. It contains two double bonds (4 π electrons) and one lone pair from the oxygen atom (2 π electrons), for a total of 6 π electrons. This satisfies Hückel’s rule for n=1 (4(1) + 2 = 6). Thus, furan is an aromatic compound.
- D (Azete): This four-membered ring is cyclic, planar, and conjugated. However, it contains 4 π electrons. This follows the 4n rule (for n=1), making it antiaromatic and highly unstable.
- E (Cyclopentadienyl cation): This five-membered ring is cyclic, planar, and conjugated but only has 4 π electrons. Like compound D, it is antiaromatic.
Therefore, compound C is the only aromatic compound among the choices.
Question 2: Which one of the following compounds is more reactive towards Electrophilic Aromatic Substitution?
Correct Answer: D (PhCH₃)
Explanation:
Electrophilic Aromatic Substitution (EAS) is a reaction in which an electrophile (an electron-seeking species) attacks an electron-rich aromatic ring. The reactivity of a substituted benzene ring in an EAS reaction depends on the nature of the substituent attached to it.
- Activating groups are electron-donating groups (EDGs). They increase the electron density of the benzene ring, making it more nucleophilic and thus more reactive towards electrophiles than benzene itself.
- Deactivating groups are electron-withdrawing groups (EWGs). They decrease the electron density of the ring, making it less nucleophilic and less reactive towards electrophiles.
Let’s classify the substituents for each compound:
- A (PhCO₂H): The carboxylic acid group (-CO₂H) is a strong electron-withdrawing group and is strongly deactivating.
- B (PhCCl₃): The trichloromethyl group (-CCl₃) is strongly electron-withdrawing due to the powerful inductive effect of the three chlorine atoms, making it strongly deactivating.
- C (PhCN): The nitrile group (-CN) is a strong electron-withdrawing group and is strongly deactivating.
- D (PhCH₃): The methyl group (-CH₃) is an electron-donating group (via induction and hyperconjugation). It is an activating group.
- E (PhCl): The chloro group (-Cl) is a halogen. Halogens are electron-withdrawing via induction but electron-donating via resonance. The inductive effect dominates, making them net deactivating (though they are ortho-, para-directing).
Comparing the options, toluene (PhCH₃) is the only compound with an activating group. All other compounds possess deactivating groups, which make their rings less reactive than benzene. Since the methyl group in toluene increases the ring’s electron density, it is the most reactive compound towards electrophilic attack.
The general order of reactivity is:
PhCH₃ (D) > PhCl (E) > PhCO₂H (A) ≈ PhCN (C) ≈ PhCCl₃ (B)thumb_upthumb_down
