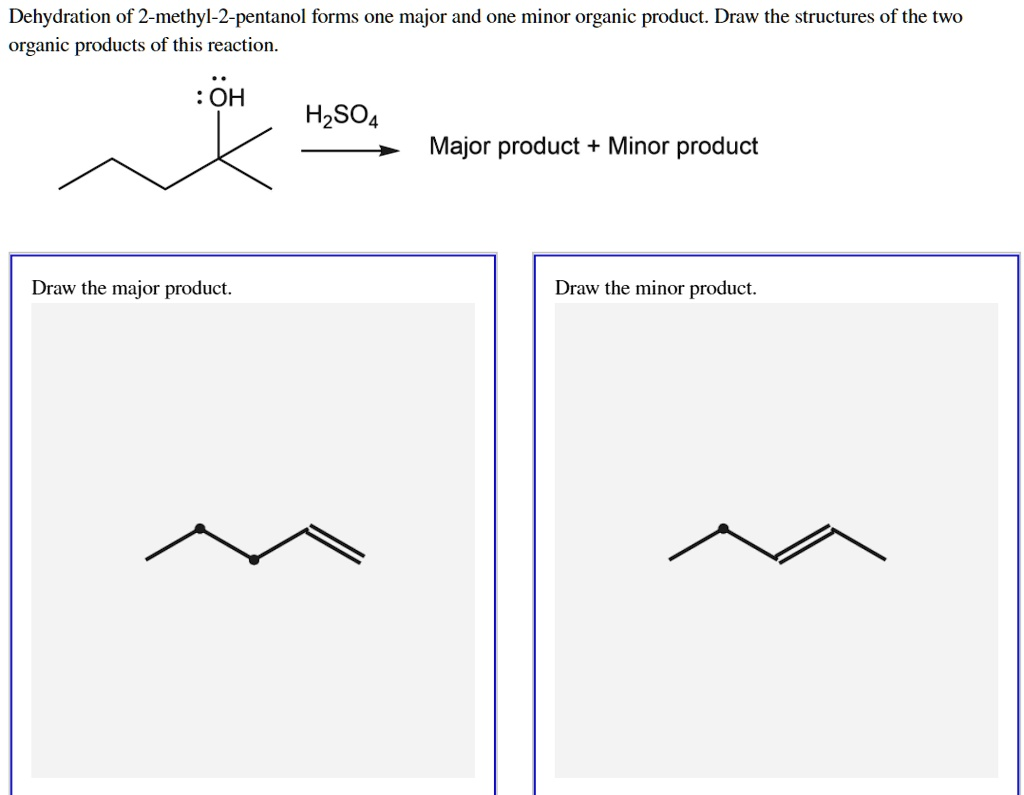
Dehydration of 2-methyl-2-pentanol forms one major and one minor organic product Draw the structures of the two organic products of this reaction. OH H2SO4 Major product Minor product Draw the major product: Draw the minor product:
The Correct Answer and Explanation is:
This is an acid-catalyzed dehydration of a tertiary alcohol, 2-methyl-2-pentanol. The reaction proceeds via an E1 elimination mechanism to form two different alkene products. The determination of the major and minor products is governed by Zaitsev’s rule.
Correct Answer:
Draw the major product.
[
{"box_2d": [655, 650, 712, 868], "label": "the major product"},
{"box_2d": [660, 139, 712, 358], "label": "the minor product"}
]content_copydownloadUse code with caution.Json
The major product is 2-methyl-2-pentene.
<br>
Draw the minor product.
The minor product is 2-methyl-1-pentene.
<br>
Explanation:
The reaction shown is the acid-catalyzed dehydration of a tertiary alcohol, 2-methyl-2-pentanol. This reaction is an elimination reaction that proceeds through an E1 mechanism.
Mechanism:
- Protonation: The first step involves the protonation of the hydroxyl (-OH) group by the strong acid, sulfuric acid (H₂SO₄). The lone pair of electrons on the oxygen atom attacks a proton (H⁺), converting the poor leaving group (-OH) into a good leaving group, a protonated water molecule (-OH₂⁺).
- Formation of a Carbocation: The protonated alcohol loses a molecule of water to form a stable tertiary carbocation intermediate, the 2-methyl-2-pentyl cation. Tertiary carbocations are relatively stable, which is why this E1 pathway is favored for tertiary alcohols.
- Deprotonation and Alkene Formation: In the final step, a weak base (such as H₂O or the conjugate base HSO₄⁻) abstracts a proton from a carbon atom adjacent (a β-carbon) to the positively charged carbon. There are two different types of β-hydrogens that can be removed in this intermediate, leading to two different alkene products.
- Path A (Minor Product): If a proton is removed from one of the hydrogens on the methyl group (C1), the product is 2-methyl-1-pentene. This alkene has two alkyl groups attached to the double-bond carbons, making it a disubstituted alkene.
- Path B (Major Product): If a proton is removed from one of the hydrogens on the methylene group (C3), the product is 2-methyl-2-pentene. This alkene has three alkyl groups attached to the double-bond carbons, making it a trisubstituted alkene.
Zaitsev’s Rule:
According to Zaitsev’s rule, in an elimination reaction that can produce more than one alkene, the major product is the more stable, more highly substituted alkene. The stability of alkenes increases with the number of alkyl substituents on the double-bond carbons (tetrasubstituted > trisubstituted > disubstituted > monosubstituted).
Since 2-methyl-2-pentene is trisubstituted, it is more thermodynamically stable than the disubstituted 2-methyl-1-pentene. Therefore, 2-methyl-2-pentene is the major product (the Zaitsev product), and 2-methyl-1-pentene is the minor product.thumb_upthumb_down
