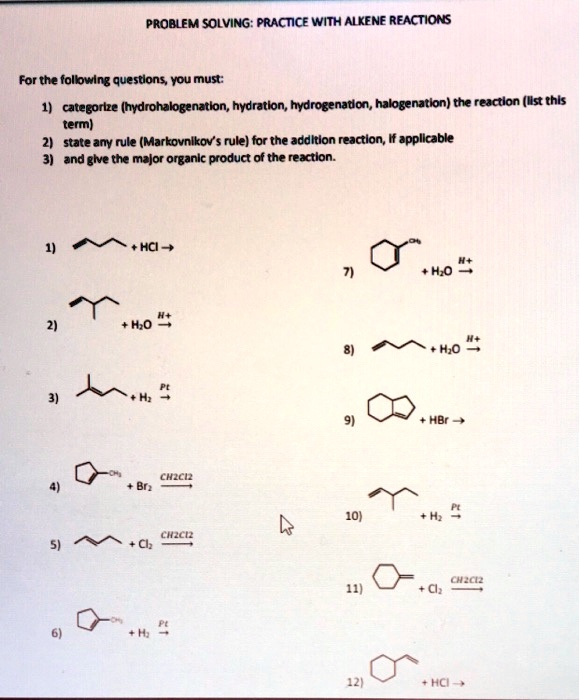
PROBLEM SOLVING: PRACTICE WITH ALKENE REACTIONS For the following questions, you must: categorize (hydrohalogenation, hydration; hydrogenation, halogenation) the reaction (Is this term), state any rule (Markovnikov’s rule) for the addition reaction applicable, and give the major organic product of the reaction: 1. HCI + H2O 2. H2O 3. F+H 4. HBr 5. CHIcE 6. 10] 7. tH 8. CMC + Cl 9. CH2C 10. HCI
The Correct Answer and Explanation is:
Here is a breakdown of the alkene reactions, their classifications, applicable rules, and major organic products:
- Alkene + HCl → Hydrohalogenation. Markovnikov’s rule applies, meaning the halide (Cl) will attach to the more substituted carbon. The major product is the corresponding alkyl chloride.
- Alkene + H₂O, H⁺ → Hydration. Markovnikov’s rule applies, favoring the attachment of the hydroxyl group (-OH) to the more substituted carbon. The major product is the corresponding alcohol.
- Alkene + H₂, Pt → Hydrogenation. No regioselectivity rule is needed, as both hydrogen atoms add across the double bond, saturating the alkene into an alkane.
- Alkene + Br₂, CH₂Cl₂ → Halogenation. No regioselectivity rule is required since bromine atoms add anti to each other, producing a vicinal dibromo compound.
- Alkene + Cl₂, CH₂Cl₂ → Halogenation. Chlorine adds across the double bond in an anti fashion, forming a vicinal dichloride.
- Alkene + H₂, Pt → Hydrogenation. Similar to reaction 3, hydrogen saturates the alkene, yielding the corresponding alkane.
- Alkene + H₂O, H⁺ → Hydration. Following Markovnikov’s rule, the hydroxyl (-OH) attaches to the more substituted carbon, forming an alcohol.
- Alkene + H₂O, H⁺ → Hydration. Produces an alcohol by Markovnikov’s rule.
- Alkene + HBr → Hydrohalogenation. Markovnikov’s rule directs bromine to the more substituted carbon, forming an alkyl bromide.
- Alkene + H₂, Pt → Hydrogenation. Converts the alkene into an alkane.
- Alkene + Cl₂, CH₂Cl₂ → Halogenation. Chlorine adds in an anti fashion, yielding a vicinal dichloride.
- Alkene + HCl → Hydrohalogenation. Markovnikov’s rule applies, forming an alkyl chloride.
Explanation:
Alkene addition reactions follow fundamental principles based on the nature of the reagent. In hydrohalogenation, hydrogen halides (HCl, HBr) add to the alkene according to Markovnikov’s rule, favoring attachment of the halogen to the more substituted carbon. Hydration reactions involve water and an acid catalyst, leading to the formation of alcohols through a similar regioselective process. Halogenation proceeds via anti addition, creating vicinal dihalides. Hydrogenation, catalyzed by metals like Pt, results in complete saturation of the double bond. These predictable patterns define organic chemistry’s core alkene transformations.
