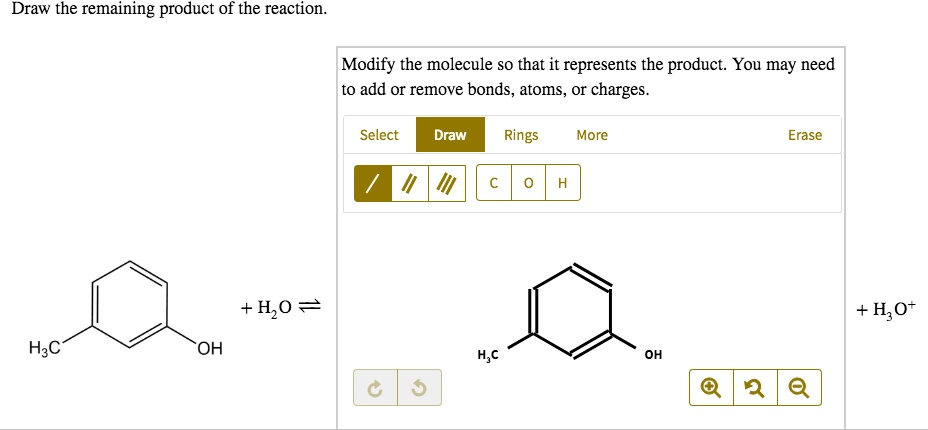
Draw the remaining product of the reaction: Modify the molecule so that it represents the product. You may need to add or remove bonds, atoms, charges. Select Draw Rings More Erase + H2O = OH + H3O- H2C H2C OH 9
The Correct Answer and Explanation is:
In this reaction, water (H₂O) acts as a reagent, interacting with the molecule to yield hydroxide (OH⁻) and hydronium (H₃O⁺). The molecular structure provided includes a benzene ring with a hydroxyl (-OH) and methyl (-CH₃) group attached. The task requires adjusting the bonds, atoms, or charges to reflect the final product.
Reaction Explanation: The presence of water implies a possible acid-base interaction. The hydroxyl (-OH) group on the benzene ring can undergo protonation or deprotonation depending on the reaction conditions. In aqueous environments, if a strong acid is present, the hydroxyl group may gain a proton, leading to the formation of hydronium ion (H₃O⁺). Conversely, if a base is involved, deprotonation may occur, forming hydroxide (OH⁻).
Considering the reaction context, the product involves a modified version of the original molecule. Water molecules facilitate proton transfer, influencing the charge distribution on the oxygen atom. The hydroxyl group transitions into a positively charged oxonium (-OH⁺), while the surrounding system balances the charge through H₃O⁺ formation.
Structural Adjustments:
- Maintain the benzene ring backbone.
- Retain the methyl (-CH₃) group attachment.
- Modify the hydroxyl (-OH) group to represent the protonated state (-OH⁺).
- Account for the formation of H₃O⁺ as the complementary species in solution.
The overall transformation involves adjusting the charge on oxygen while ensuring the hydronium ion (H₃O⁺) forms in equilibrium with the molecular system. This reflects the reaction’s proton transfer dynamics. The finalized molecule consists of a benzene ring with methyl (-CH₃) and a positively charged hydroxyl (-OH⁺) group, balancing the charge distribution with H₃O⁺ in solution.
