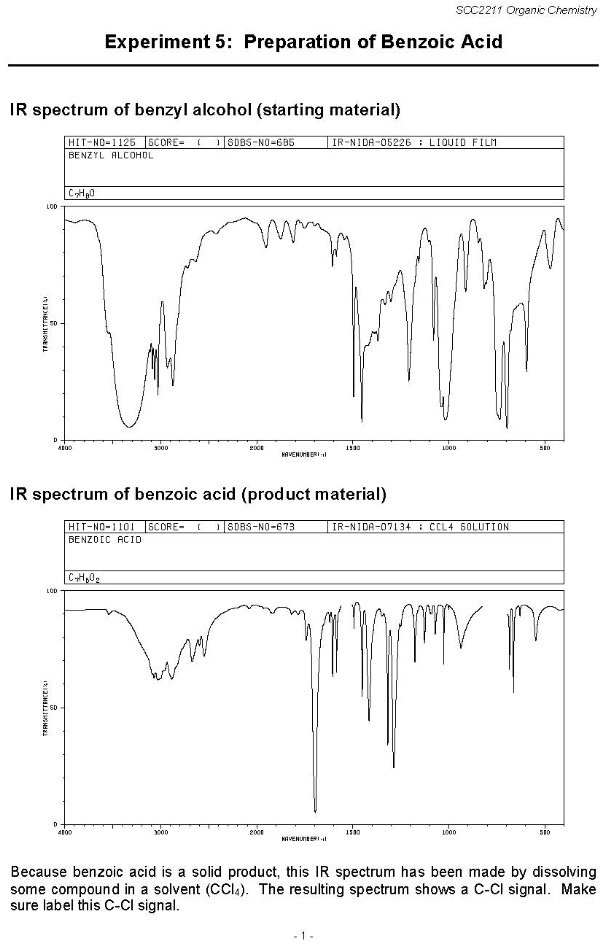
IR spectrum of benzyl alcohol (starting material) HIT-NO-1125 SCORE- ( ) SDBS-NO-685 IR-NIDA-05226 : LIQUID FILM BENZYL ALCOHOL
TRANSHETTANCE 100 50 0 4000 3000 2000 1500 1000 500 WAVENUMBER(1-1) IR spectrum of benzoic acid (product material) HIT-NO-1101 SCORE- ( ) SDBS-NO-673 IR-NIDA-07134 : CCL4 SOLUTION BENZOIC ACID
TRANSHETTANCE 100 50 0 4000 3000 2000 1500 1000 500 WAVENUMBER(1-1) Because benzoic acid is a solid product, this IR spectrum has been made by dissolving some compound in a solvent (CCl
). The resulting spectrum shows a C-Cl signal. Make sure label this C-Cl signal.
SOC2211 Organic Chemistry Experiment 5: Preparation of Benzoic Acid IR spectrum of benzyl alcohol (starting material) HIT-NO-1125 SCORE- ( ) SDBS-NO-685 IR-NIDA-05226 : LIQUID FILM BENZYL ALCOHOL
TRANSHETTANCE 100 50 0 4000 3000 2000 1500 1000 500 WAVENUMBER(1-1) IR spectrum of benzoic acid (product material) HIT-NO-1101 SCORE- ( ) SDBS-NO-673 IR-NIDA-07134 : CCL4 SOLUTION BENZOIC ACID
TRANSHETTANCE 100 50 0 4000 3000 2000 1500 1000 500 WAVENUMBER(1-1) Because benzoic acid is a solid product, this IR spectrum has been made by dissolving some compound in a solvent (CCl
). The resulting spectrum shows a C-Cl signal. Make sure label this C-Cl signal.
The Correct Answer and Explanation is:
The infrared (IR) spectra provided show the transition from benzyl alcohol to benzoic acid. The spectral features of benzyl alcohol include a broad O-H stretching vibration between 3200 and 3600 cm⁻¹, which is characteristic of hydroxyl functional groups. Additionally, the C-H stretching vibrations in the range of 2800 to 3100 cm⁻¹ indicate the presence of alkyl and aromatic components. The C-O stretching signal appears near 1050 to 1250 cm⁻¹, which is typical for alcohols.
In the IR spectrum of benzoic acid, the most significant change is the disappearance of the O-H signal associated with alcohols and the emergence of a strong and broad O-H stretching vibration due to carboxylic acid between 2500 and 3500 cm⁻¹. The presence of a sharp and intense C=O stretching vibration near 1700 cm⁻¹ confirms the formation of the carboxyl functional group. Additionally, the C-O stretching modes appear around 1250 cm⁻¹, contributing to the identification of carboxylic acid functionality. Since benzoic acid was dissolved in carbon tetrachloride (CCl₄) for this analysis, the resulting spectrum includes a C-Cl stretching band, typically found between 600 and 800 cm⁻¹.
The transformation from benzyl alcohol to benzoic acid is an oxidation process where the primary alcohol is converted into a carboxylic acid. This conversion introduces a distinct shift in spectral features, particularly the disappearance of alcohol-related peaks and the appearance of strong carboxyl absorptions. The IR spectrum is a valuable tool in verifying this transformation, as the key differences indicate the successful oxidation of benzyl alcohol to benzoic acid. Correctly labeling the C-Cl signal is necessary to differentiate solvent contributions from the actual structural features of the product.
