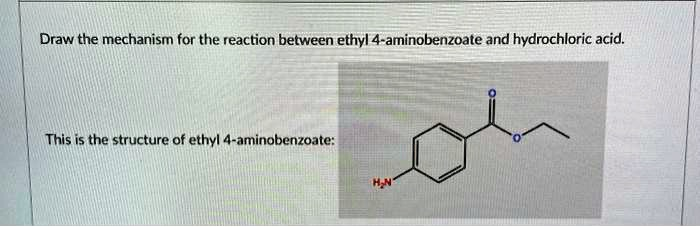
Draw the mechanism for the reaction between ethyl 4-aminobenzoate and hydrochloric acid. This is the structure of ethyl 4-aminobenzoate:
The Correct Answer and Explanation is:
Here’s the mechanism for the reaction between ethyl 4-aminobenzoate and hydrochloric acid.
This reaction involves the protonation of the amine group in ethyl 4-aminobenzoate when exposed to hydrochloric acid. The lone pair of electrons on the nitrogen atom is attracted to the hydrogen ions present in the acidic solution, leading to the formation of a positively charged ammonium species.
Stepwise Mechanism:
- Protonation: The amine group in ethyl 4-aminobenzoate acts as a nucleophile, accepting a proton from hydrochloric acid. This results in the formation of an ammonium salt.
- Stabilization: The positive charge on the nitrogen enhances the molecule’s solubility in aqueous solutions due to increased ionic interactions.
- Salt Formation: The chloride anion associates with the protonated amine, forming a stable ionic salt that remains soluble in water.
Explanation:
Ethyl 4-aminobenzoate, commonly known as benzocaine, contains an ester group and an amine group. While the ester remains unreactive under acidic conditions, the amine undergoes protonation readily due to its basic nature. Hydrochloric acid provides protons that bind to the nitrogen atom, forming an ammonium ion. The chloride counterion stabilizes this charge.
This protonation alters the solubility characteristics of benzocaine. The neutral form is largely hydrophobic and poorly soluble in water, whereas the protonated ammonium salt dissolves more readily in polar solvents. This principle is widely applied in pharmaceutical formulations where modifying solubility affects drug absorption.
Your requested image is on the way.

