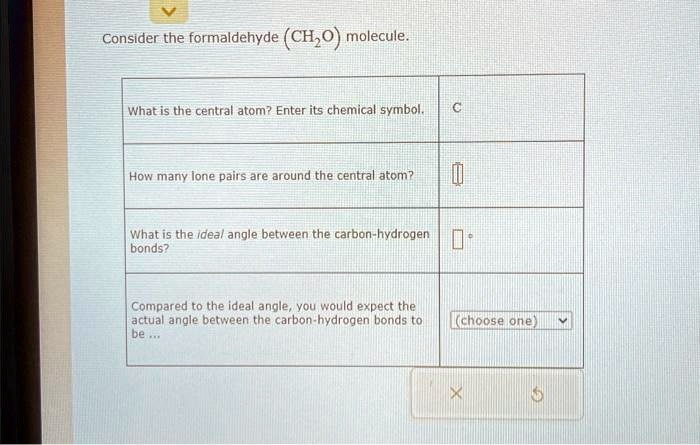
Consider the formaldehyde (CH2O
) molecule. What is the central atom? Enter its chemical symbol. C How many lone pairs are around the central atom? What is the ideal angle between the carbon-hydrogen bonds? Compared to the ideal angle, you would expect the actual angle between the carbon-hydrogen bonds to be
The Correct Answer and Explanation is:
Correct Answers:
- What is the central atom?
C - How many lone pairs are around the central atom?
0 - What is the ideal angle between the carbon-hydrogen bonds?
120° - Compared to the ideal angle, you would expect the actual angle between the carbon-hydrogen bonds to be:
Slightly less
Explanation
Formaldehyde (CH₂O) is a simple organic molecule with carbon as the central atom. In this structure, carbon forms a double bond with the oxygen atom and single bonds with two hydrogen atoms. As a result, carbon is bonded to three regions of electron density: one double bond and two single bonds. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, these three regions arrange themselves as far apart as possible, forming a trigonal planar geometry.
In a trigonal planar structure, the ideal bond angles are 120°. This would be the angle between any two bonds, including the two carbon-hydrogen bonds. Since the central carbon is forming only bonds and has no lone pairs, there are no additional repulsions from non-bonding electron pairs that would drastically alter these angles. Therefore, the ideal angle between the hydrogen atoms is 120°.
However, real molecular geometry often deviates slightly from these ideal angles due to unequal repulsions. In CH₂O, the carbon-oxygen double bond occupies more space than a single bond because of its higher electron density. This causes the bond angles adjacent to it (the C–H bonds) to be pushed slightly closer together. Thus, the actual H–C–H bond angle ends up being slightly less than 120°, while the H–C–O angles become slightly larger.
There are zero lone pairs on the central carbon because it already has a complete octet with its four shared pairs (two from the double bond and one from each hydrogen). This absence of lone pairs means that any deviation from ideal geometry comes from differences in bond repulsion, not electron lone pair repulsion.
This makes formaldehyde an excellent example of how double bonds and bond repulsion influence actual molecular shape.
