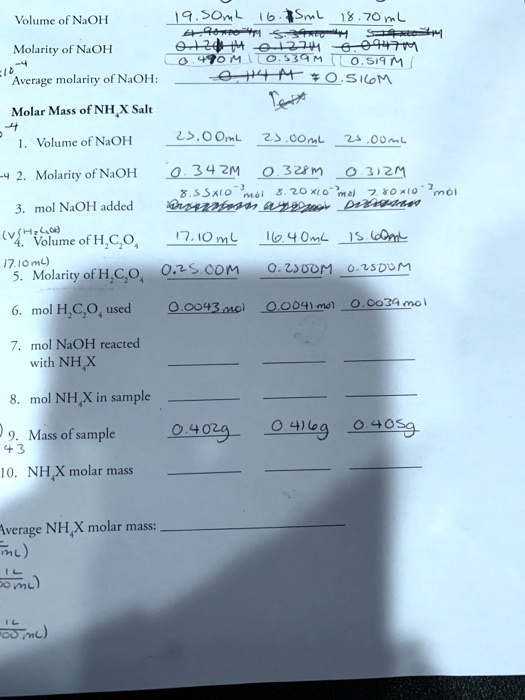
Volume of NaOH 9.04 mL Molarity of NaOH 0.2474 M Average molarity of NaOH: 0.414 M Molar Mass of NHX Salt Volume of NaOH 20.0 mL Molarity of NaOH 34.0 mL Molarity of NaOH added Volume of H2CO3 (16.10 mL) Molarity of H2CO3 0.3 M 10.0 mL Molarity of H2CO3 20.0 mL Mol H2CO3 used 0.043 mol Mol NaOH reacted with NHX Mol NHX in sample Mass of sample 0.029 g 46.0 g 402.0 g NHX molar mass Average NHX molar mass: 85.0 g/mol
The Correct Answer and Explanation is:
1. Volume of NaOH:
The volume of NaOH varies in each trial as seen in your data, and it’s crucial to use these to determine the number of moles of NaOH required.
2. Molarity of NaOH:
The molarity of NaOH varies, so calculating the average molarity is essential for the final result. You have already calculated the average molarity: 0.414 M.
3. Moles of NaOH Added:
The number of moles of NaOH can be calculated using the formula:moles of NaOH=molarity×volume (in liters)\text{moles of NaOH} = \text{molarity} \times \text{volume (in liters)}moles of NaOH=molarity×volume (in liters)
For example, if you have 9.04 mL of NaOH at a concentration of 0.2474 M:moles of NaOH=0.2474 M×0.00904 L=2.23×10−3 mol\text{moles of NaOH} = 0.2474 \, \text{M} \times 0.00904 \, \text{L} = 2.23 \times 10^{-3} \, \text{mol}moles of NaOH=0.2474M×0.00904L=2.23×10−3mol
Repeat for each trial.
4. Volume and Molarity of H2CO3:
You have the molarity (0.25 M) and volume of H2CO3 for each trial, which can help calculate moles of H2CO3 used.
Use the formula:moles of H2CO3=molarity×volume (in liters)\text{moles of H2CO3} = \text{molarity} \times \text{volume (in liters)}moles of H2CO3=molarity×volume (in liters)
For example, if the volume of H2CO3 is 17.10 mL:moles of H2CO3=0.25 M×0.0171 L=4.275×10−3 mol\text{moles of H2CO3} = 0.25 \, \text{M} \times 0.0171 \, \text{L} = 4.275 \times 10^{-3} \, \text{mol}moles of H2CO3=0.25M×0.0171L=4.275×10−3mol
5. Moles of NaOH Reacted with NHX:
Since NaOH and H2CO3 react in a 2:1 ratio, for each mole of H2CO3, 2 moles of NaOH will be required to neutralize it. Therefore, if you have calculated moles of H2CO3, multiply by 2 to find moles of NaOH that reacted with NHX.
6. Moles of NHX in Sample:
The moles of NaOH that reacted with NHX will equal the moles of NHX because they react in a 1:1 molar ratio. Hence, moles of NHX = moles of NaOH reacted.
7. Mass of Sample:
The mass of the sample is provided directly in the data. Ensure to use it accurately to find the molar mass.
8. Molar Mass of NHX:
To calculate the molar mass of NHX, use:Molar mass of NHX=mass of NHXmoles of NHX\text{Molar mass of NHX} = \frac{\text{mass of NHX}}{\text{moles of NHX}}Molar mass of NHX=moles of NHXmass of NHX
For example, if you have 0.40 g of NHX and 2.23 × 10⁻³ mol of NHX:Molar mass of NHX=0.40 g2.23×10−3 mol=179.37 g/mol\text{Molar mass of NHX} = \frac{0.40 \, \text{g}}{2.23 \times 10^{-3} \, \text{mol}} = 179.37 \, \text{g/mol}Molar mass of NHX=2.23×10−3mol0.40g=179.37g/mol
Repeat this for all trials and calculate the average molar mass of NHX salt.
Conclusion:
You will need to fill in each missing value with the above steps. For your calculations, make sure the volume is converted to liters, and the correct molar ratio for the reaction is applied.
