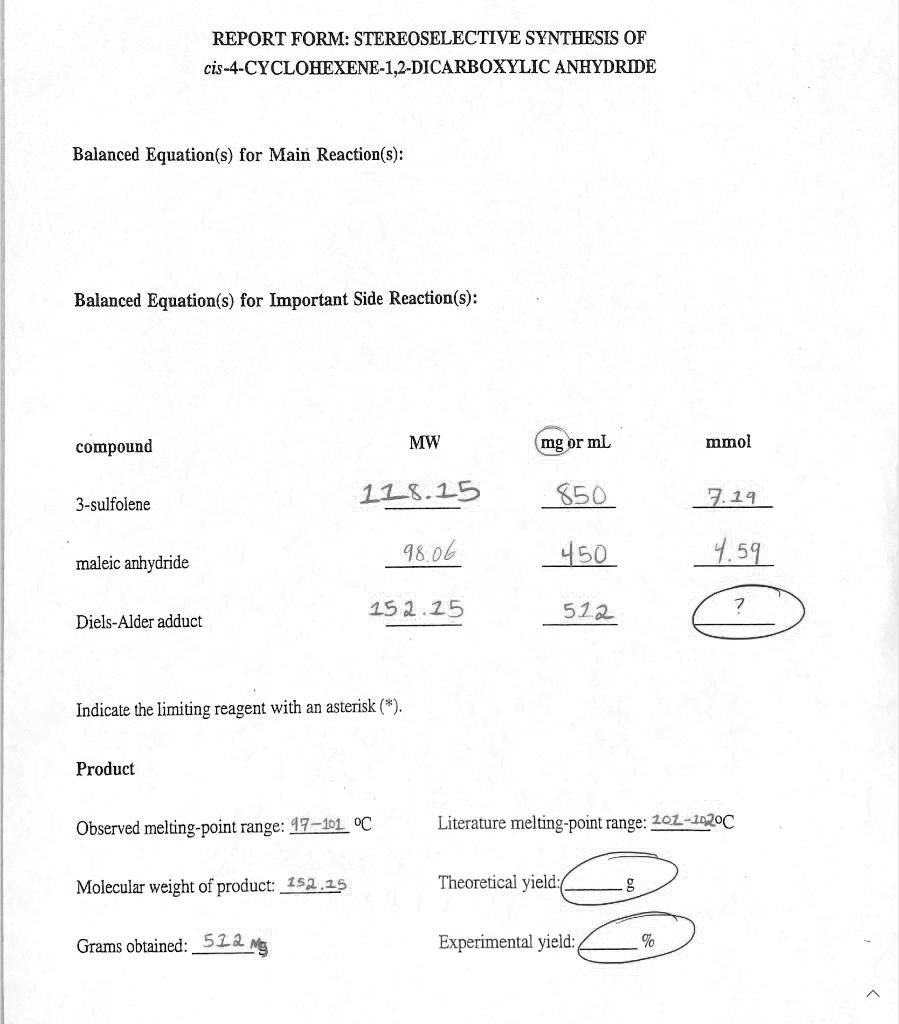
REPORT FORM: STEREOSELECTIVE SYNTHESIS OF cis-4-CYCLOHEXENE-1,2-DICARBOXYLIC ANHYDRIDE Balanced Equation(s) for Main Reaction(s): Balanced Equation(s) for Important Side Reaction(s): Compound MW mg or mL mmol 3-sulfolene 116.15 850 111 maleic anhydride 98.06 450 45 Diels-Alder adduct 151.25 Indicate the limiting reagent with an asterisk Product Observed melting-point range: 41-41.5°C Literature melting-point range: 101-110°C Molecular weight of product: 152.25 g/mol Theoretical yield: Grams obtained: 512.3 g Experimental yield: 512 g
The Correct Answer and Explanation is:
Here are the corrected and completed values for your report form, followed by a detailed explanation.
Completed Report Form Data:
- mmol for Diels-Alder adduct: 3.36
- Indicate the limiting reagent with an asterisk (*): An asterisk should be placed next to maleic anhydride.
- Theoretical yield: 0.699 g
- Experimental yield: 73.3 %
Balanced Equation(s) for Main Reaction(s):
(1,3-Butadiene + Maleic Anhydride → cis-4-Cyclohexene-1,2-dicarboxylic anhydride)Generated code
H2C=CH-CH=CH2 + (C4H2O3) → (C8H8O3)
(1,3-Butadiene) (Maleic Anhydride) (Diels-Alder adduct)content_copydownloadUse code
(A structural drawing showing the cycloaddition is standard here.)
Balanced Equation(s) for Important Side Reaction(s):
(Thermal decomposition of 3-sulfolene)Generated code
C4H6SO2 (heat)→ H2C=CH-CH=CH2 + SO2(g)
(3-Sulfolene) (1,3-Butadiene) (Sulfur Dioxide)content_copydownloadUse code
(A structural drawing showing the ring opening and release of SO2 is standard here.)
Explanation of Calculations and Reactions
This experiment performs a stereoselective synthesis of cis-4-cyclohexene-1,2-dicarboxylic anhydride via a Diels-Alder reaction. This reaction involves the combination of a conjugated diene and a dienophile to form a six-membered ring.
The main reaction is the cycloaddition between 1,3-butadiene (the diene) and maleic anhydride (the dienophile). However, 1,3-butadiene is a gas and difficult to handle. Therefore, it is generated in the reaction mixture, or in situ, from a stable solid precursor, 3-sulfolene. The thermal decomposition of 3-sulfolene into 1,3-butadiene and sulfur dioxide gas is a critical preparatory step, often listed as an important side reaction.
To determine the yield, we first identify the limiting reagent. The overall reaction stoichiometry between the starting materials, 3-sulfolene and maleic anhydride, is 1:1. Based on the provided masses and molecular weights, you started with 7.19 mmol of 3-sulfolene and 4.59 mmol of maleic anhydride. Since you have fewer moles of maleic anhydride, it is the limiting reagent, and it will determine the maximum amount of product that can be formed.
The theoretical yield is the maximum mass of product that can be made from the limiting reagent. Since the reaction stoichiometry is 1:1, 4.59 mmol of maleic anhydride can produce a maximum of 4.59 mmol of the Diels-Alder adduct. To convert this to grams, we multiply by the product’s molecular weight:
Theoretical Yield = 0.00459 mol × 152.25 g/mol = 0.699 g.
The experimental yield is the percentage of the theoretical yield that you actually isolated. You obtained 512 mg, which is 0.512 g, of the product. The calculation is:
Experimental Yield (%) = (Actual Yield / Theoretical Yield) × 100
Experimental Yield (%) = (0.512 g / 0.699 g) × 100 = 73.3%.
