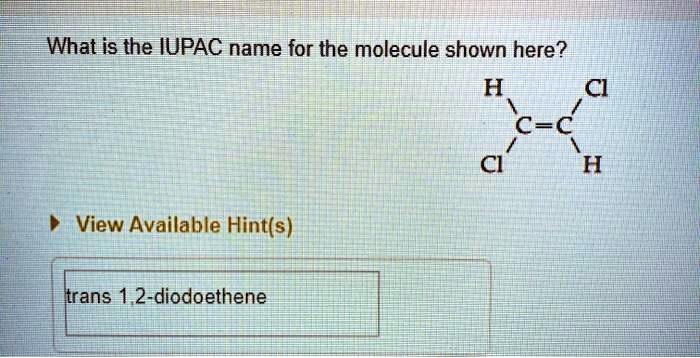
What is the IUPAC name for the molecule shown here? H Cl C=C Cl H
The Correct Answer and Explanation is:
The correct IUPAC name for the molecule is trans-1,2-dichloroethene.
Here is a step-by-step explanation of how to determine the name:
- Identify the Parent Chain: The core of the molecule is a carbon chain containing a double bond (C=C). The longest chain that includes this double bond consists of two carbon atoms. The alkane with two carbons is ethane. Because of the double bond, the suffix changes from “-ane” to “-ene”. Therefore, the parent name of the molecule is “ethene”.
- Identify and Name Substituents: The molecule has two substituent groups attached to the carbon atoms. These are both chlorine (Cl) atoms. The IUPAC prefix for a chlorine substituent is “chloro”. Since there are two chlorine atoms, we use the numerical prefix “di-“. This gives us the substituent part of the name: “dichloro”.
- Number the Carbon Chain and Locate Substituents: For ethene, we number the two carbons as 1 and 2. One chlorine atom is attached to carbon 1, and the other is attached to carbon 2. We indicate their positions with numbers, resulting in “1,2-dichloro”.
- Determine the Stereochemistry: The presence of the double bond restricts rotation, which can lead to different spatial arrangements called stereoisomers. We need to specify this arrangement.
- In the given structure, the two hydrogen (H) atoms are on opposite sides of the imaginary line drawn through the double bond.
- Similarly, the two chlorine (Cl) atoms are on opposite sides of the double bond.
- When identical groups (or groups of highest priority) are on opposite sides of the double bond, the prefix “trans” is used. The alternative is “cis”, where the groups would be on the same side.
Combining these parts gives the complete IUPAC name: trans-1,2-dichloroethene. The name shown in the text box of the image, “trans 1,2-diiodoethene”, is incorrect because the atoms are chlorine (Cl), not iodine (I).thumb_upthumb_down
