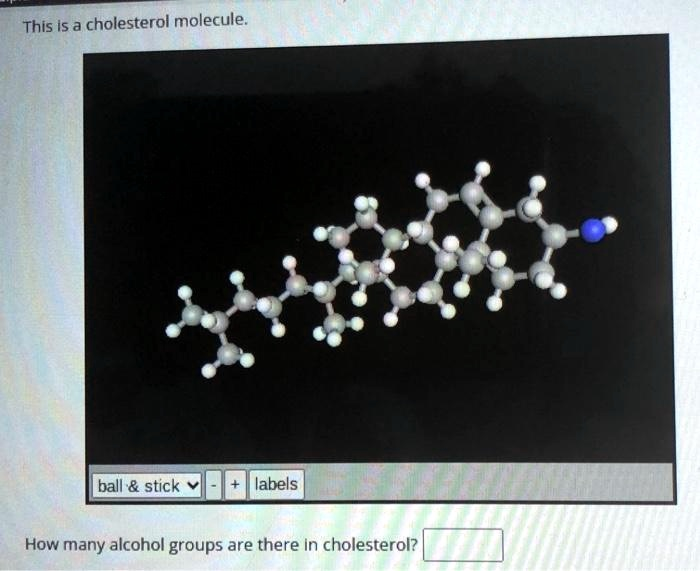
This is a cholesterol molecule. ball & stick + labels How many alcohol groups are there in cholesterol?
The Correct Answer and Explanation is:
A cholesterol molecule contains one alcohol group.
An alcohol functional group, more formally called a hydroxyl group, consists of an oxygen atom covalently bonded to a hydrogen atom (-OH). This group is the defining feature that classifies a molecule as an alcohol. Cholesterol is specifically categorized as a sterol, a term derived from “steroid alcohol,” which indicates the presence of this very functional group within its steroid structure.
The chemical architecture of cholesterol is based on a rigid four-ring carbon skeleton known as a steroid nucleus. Attached to this nucleus are various side groups, including a hydrocarbon tail. The crucial functional group that gives cholesterol its alcohol properties is a single hydroxyl group located at a specific position, the third carbon atom (C3) of the first ring (the A-ring).
This single hydroxyl group is vital for cholesterol’s biological functions. It imparts a small, polar region onto the otherwise large, nonpolar molecule. This dual characteristic, known as being amphipathic, allows cholesterol to embed itself correctly within the phospholipid bilayer of cell membranes, where it helps regulate membrane fluidity and permeability. This hydroxyl group is also the reactive site for many biochemical transformations, such as its conversion into cholesterol esters for transport in the bloodstream or its use as the starting point for the body to synthesize essential steroid hormones, bile acids, and vitamin D.
While the provided image is labeled as a cholesterol molecule, it inaccurately depicts an amine group (-NH2), indicated by the blue nitrogen atom, in place of the hydroxyl group. A correct model would show a red oxygen atom. Nevertheless, based on the actual chemical structure of cholesterol, it contains precisely one alcohol group.
