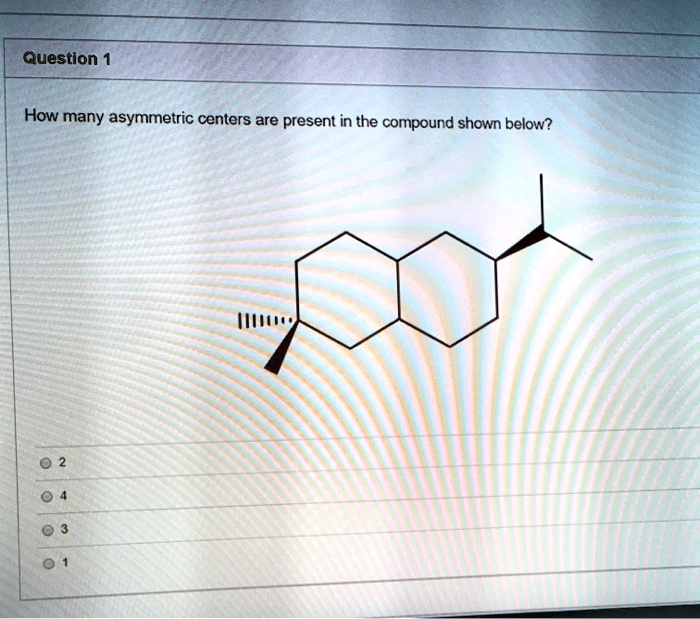
How many asymmetric centers are present in the compound shown below? 2 4 3 1
The Correct Answer and Explanation is:
The correct answer is 3.
An asymmetric center, or a chiral center, is a carbon atom that is bonded to four different groups. To determine the number of asymmetric centers in the provided molecule, we need to inspect each carbon atom to see if it meets this criterion.
The molecule shown is a substituted decalin, which has a fused two-ring system. Let’s analyze the potential chiral centers:
- The carbon with the gem-dimethyl group: This carbon atom, located on the left ring, is attached to two methyl groups (one indicated by a solid wedge and the other by a dashed wedge), and two other carbon atoms within the ring. An essential requirement for an asymmetric center is having four different substituents. Since this carbon is bonded to two identical methyl groups, it is not an asymmetric center.
- The carbon with the isopropyl group: This carbon atom, on the right ring, is bonded to four distinct groups:
- An isopropyl group (-CH(CH₃)₂).
- An implied hydrogen atom.
- The carbon-carbon bond leading clockwise around the ring system.
- The carbon-carbon bond leading counter-clockwise around the ring system.
The two pathways around the fused ring system are different from each other due to the presence of the gem-dimethyl group on the other ring. Because all four attached groups are different, this carbon is an asymmetric center.
- The two bridgehead carbons: These are the carbons where the two rings are joined.
- The bridgehead carbon that is directly bonded to both the gem-dimethyl-substituted carbon and the isopropyl-substituted carbon is one potential center. It is bonded to a hydrogen atom, the other bridgehead carbon, the gem-dimethyl-substituted carbon, and the isopropyl-substituted carbon. These four groups are all structurally different, making this bridgehead carbon an asymmetric center.
- The second bridgehead carbon is bonded to a hydrogen atom, the first bridgehead carbon, and two other carbons (CH₂ groups) in the rings. The paths leading away from this carbon through the two CH₂ groups are not equivalent. One path leads toward the ring with the gem-dimethyl group, and the other leads toward the ring with the isopropyl group. Since these two pathways are different, this bridgehead carbon is also an asymmetric center.
Therefore, the compound has a total of three asymmetric centers: the carbon bearing the isopropyl group and both bridgehead carbons.
