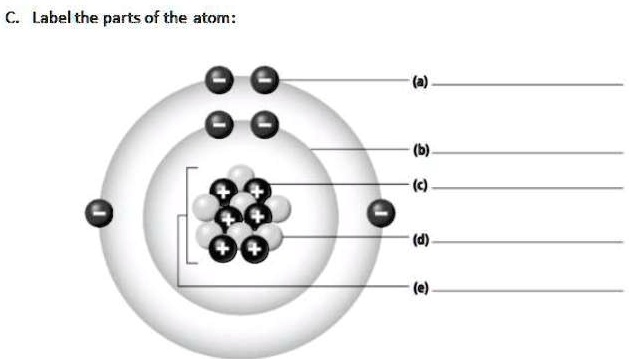
Label the different parts of the atom. Label the parts of the atom
The Correct Answer and Explanation is:
Here are the labels for the different parts of the atom shown in the diagram:
(a) Electron
(b) Electron Shell (or Valence Shell/Energy Level)
(c) Proton
(d) Neutron
(e) Nucleus
Explanation of the Parts of an Atom
The image displays a Bohr model of a neutral atom, illustrating its fundamental components. An atom is the smallest unit of matter that retains the properties of a chemical element. It consists of two main regions: a central nucleus and an outer region occupied by electrons.
(e) Nucleus: The label (e) points to the dense, central core of the atom, known as the nucleus. The nucleus contains almost all of the atom’s mass and is composed of two types of subatomic particles: protons and neutrons.
(c) Proton: The particle labeled (c) is a proton. Protons are distinguished by their positive (+) electrical charge. The number of protons in an atom’s nucleus is its atomic number, which uniquely identifies the element. In this diagram, there are six protons, meaning this is an atom of Carbon.
(d) Neutron: The particle labeled (d) is a neutron. Neutrons are electrically neutral, having no charge. They are bound together with protons in the nucleus by the strong nuclear force. While the number of protons defines an element, the number of neutrons can vary, resulting in different isotopes of that element. This atom has six neutrons.
(a) Electron: The particle labeled (a) is an electron. Electrons are subatomic particles with a negative (-) electrical charge. They are significantly smaller and lighter than protons and neutrons. Electrons do not have a fixed position but exist in a cloud, orbiting the nucleus in specific regions called energy levels or shells. In a neutral atom, the number of electrons is equal to the number of protons, balancing the overall charge. This atom has six electrons.
(b) Electron Shell: The line for (b) indicates an electron shell, also known as an energy level. These shells are the specific paths or regions around the nucleus where electrons are most likely to be found. The outermost shell, which is pointed to here, is called the valence shell. The electrons in this shell (valence electrons) are crucial as they are involved in forming chemical bonds with other atoms.
