For each of the following molecules or ions that contain sulfur, write the Lewis structure(s), predict the molecular structure (including bond angles), and give the expected hybrid orbitals for sulfur: SO2, SO3, S2O, S2O3.
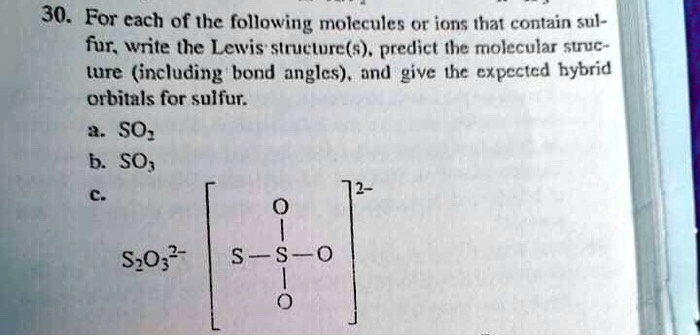
The Correct Answer and Explanation is:
Here are the Lewis structures, molecular structures, and hybrid orbitals for sulfur in the requested molecules and ion.
a. SO₂ (Sulfur Dioxide)
- Lewis Structure: Sulfur dioxide has 18 valence electrons. The Lewis structure for SO₂ is best represented by two resonance structures. In these structures, the central sulfur atom is double-bonded to one oxygen atom and single-bonded to the other, and
