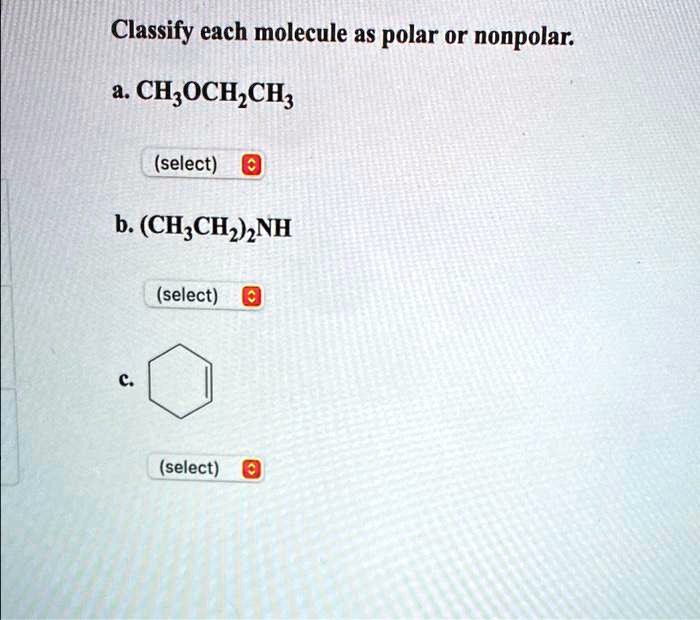
Classify each molecule as polar or nonpolar. a. CH
OCH
CH
(select) b. (CH
CH
)
NH (select) c. (select)
The Correct Answer and Explanation is:
Here are the correct classifications for each molecule, along with a detailed explanation.
a. CH₃OCH₂CH₃: Polar
b. (CH₃CH₂)₂NH: Polar
c. Cyclohexene: Nonpolar
Explanation
Molecular polarity describes the distribution of electrical charge within a molecule. A molecule’s polarity is determined by both the polarity of its individual bonds and its overall geometric shape. If a molecule contains polar bonds and has an asymmetrical shape, it will have a net dipole moment and be considered polar. Conversely, a molecule with only nonpolar bonds or one with symmetrically arranged polar bonds will be nonpolar.
a. CH₃OCH₂CH₃ (Ethyl methyl ether)
This molecule is an ether. It contains polar C-O bonds because oxygen is significantly more electronegative than carbon. This difference in electronegativity creates a dipole moment for each C-O bond, with a partial negative charge on the oxygen atom and partial positive charges on the adjacent carbon atoms. The oxygen atom also has two lone pairs of electrons, which forces the molecule into a bent geometry around the oxygen atom, similar to water. Because of this asymmetrical, bent shape, the individual bond dipoles do not cancel each other out. This results in an overall net dipole moment for the molecule, making ethyl methyl ether polar.
b. (CH₃CH₂)₂NH (Diethylamine)
This molecule is a secondary amine. It contains a nitrogen atom, which is more electronegative than both carbon and hydrogen. This creates polar C-N bonds and a polar N-H bond. The nitrogen atom also has one lone pair of electrons. Due to its three bonding pairs and one lone pair, the nitrogen atom has a trigonal pyramidal geometry. This asymmetrical shape means the individual bond dipoles do not cancel. The lone pair and the polar bonds create a net dipole moment, with the negative end centered on the nitrogen atom. Therefore, diethylamine is a polar molecule.
c. Cyclohexene
This molecule is a hydrocarbon, which means it is composed entirely of carbon and hydrogen atoms. The electronegativity difference between carbon and hydrogen is very small, so C-H bonds are considered nonpolar. The C-C bonds are, of course, perfectly nonpolar as they connect identical atoms. Since the molecule is built from nonpolar bonds, there is no significant separation of charge across the structure. The molecule has an even distribution of electron density, resulting in no net dipole moment. Consequently, cyclohexene is classified as a nonpolar molecule
