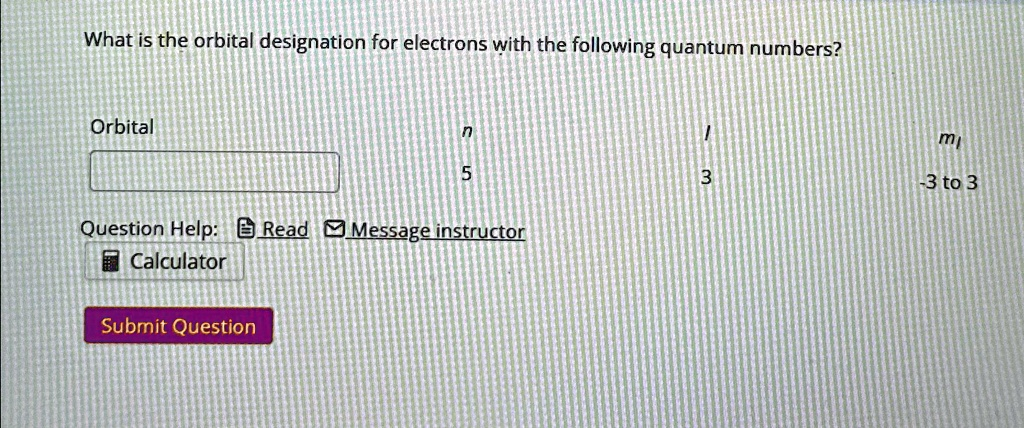
What is the orbital designation for electrons with the following quantum numbers? Orbital n / m 5 3 -3 to 3 Question Help:Read Message instructor Calculator Submit Question
The Correct Answer and Explanation is:
Correct Answer: 5f
Explanation
The orbital designation for an electron is determined by its principal quantum number (n) and its angular momentum quantum number (l). These numbers are part of a set of four quantum numbers that describe the unique state of an electron in an atom, according to the Schrödinger wave equation.
- The Principal Quantum Number (n): This number specifies the principal energy level, or shell, of an electron. It can be any positive integer (1, 2, 3, etc.). A higher value of n indicates a higher energy level and a greater average distance from the nucleus. In this problem, n = 5, which means the electrons are in the fifth energy shell. This number becomes the numerical prefix in the orbital designation.
- The Angular Momentum Quantum Number (l): This number defines the shape of the orbital and designates the subshell within the principal shell. The value of l can range from 0 to n-1. Each value of l corresponds to a specific letter used to name the subshell:
- l = 0 corresponds to the s subshell.
- l = 1 corresponds to the p subshell.
- l = 2 corresponds to the d subshell.
- l = 3 corresponds to the f subshell.
In this case, l = 3, which means the electrons are in an f subshell.
- The Magnetic Quantum Number (m_l): This number describes the orientation of the orbital in space. Its possible values range from -l to +l, including 0. For l = 3, the possible values for m_l are -3, -2, -1, 0, +1, +2, and +3. The problem statement gives the range m_l = -3 to 3, which encompasses all seven possible orientations for an f subshell. This confirms the consistency of the quantum numbers provided.
To find the orbital designation, we combine the principal quantum number (n) with the letter corresponding to the angular momentum quantum number (l). With n = 5 and l = 3 (which corresponds to ‘f’), the orbital designation is 5f. This represents the 5f subshell, which contains seven distinct orbitals.
