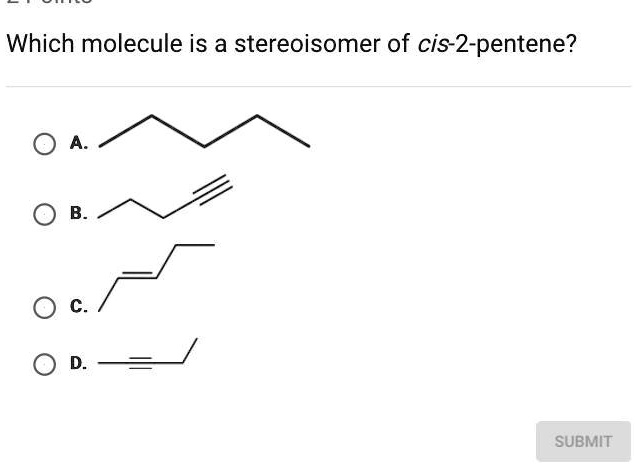
Which molecule is a stereoisomer of cis-2-pentene
The Correct Answer and Explanation is:
The correct answer is C.
Explanation
To identify a stereoisomer of cis-2-pentene, we first need to understand the definitions of isomers and stereoisomers. Isomers are molecules that share the same molecular formula but have different arrangements of atoms. Stereoisomers are a specific type of isomer where the molecules have both the same molecular formula and the same connectivity (the sequence of bonded atoms), but they differ in the three-dimensional orientation of their atoms in space.
Let’s analyze the starting molecule, cis-2-pentene:
- “Pent” indicates a chain of five carbon atoms.
- “ene” signifies the presence of a carbon-carbon double bond.
- “2-“ specifies that the double bond is located between the second and third carbon atoms.
- The molecular formula for any pentene is C₅H₁₀.
- “cis” describes the geometry around the double bond. It means that the two substituent groups on the carbons of the double bond (in this case, a methyl group on carbon 2 and an ethyl group on carbon 3) are positioned on the same side of the double bond.
A stereoisomer of cis-2-pentene must therefore also be a 2-pentene molecule with the formula C₅H₁₀. The only difference will be its spatial arrangement. The alternative arrangement for a double bond is trans, where the substituent groups are on opposite sides. Thus, we are looking for the structure of trans-2-pentene.
Now let’s examine the given options:
- A. Pentane: This molecule is an alkane, containing only single bonds. Its molecular formula is C₅H₁₂. Since its molecular formula is different from C₅H₁₀, it is not an isomer of 2-pentene.
- B. 1-Pentyne: This molecule is an alkyne, containing a triple bond at the first carbon. Its molecular formula is C₅H₈. This formula is different from C₅H₁₀, so it cannot be an isomer of 2-pentene. Molecules with different formulas are simply different compounds.
- C. trans-2-Pentene: This molecule has a five-carbon chain with a double bond between the second and third carbons. Its molecular formula is C₅H₁₀. The structure shows the methyl group (at C2) and the ethyl group (at C3) on opposite sides of the double bond. This is the definition of a trans isomer. Since it has the same molecular formula and connectivity as cis-2-pentene but differs in its spatial geometry, it is a stereoisomer. Specifically, cis and trans isomers are a type of diastereomer known as geometric isomers.
- D. 2-Pentyne: This molecule is an alkyne with a triple bond between the second and third carbons. Its molecular formula is C₅H₈. Like option B, it has a different molecular formula and is not an isomer of 2-pentene.
Therefore, the only molecule that fits the definition of a stereoisomer of cis-2-pentene is option C, which is trans-2-pentene.
